Rasagiline tablet
A technology of rasagiline tablet and rasagiline mesylate, applied in the field of pharmaceutical preparations, can solve the problems of many production processes, high cost, complicated prescription and the like, and achieve the effects of good stability and good long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
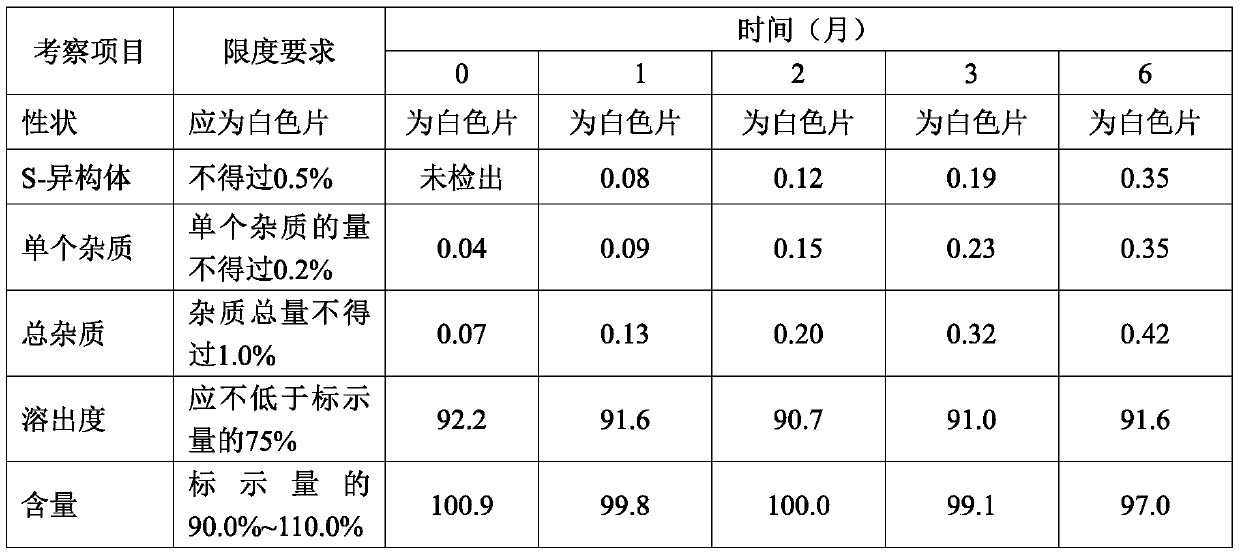
Examples
Embodiment 1
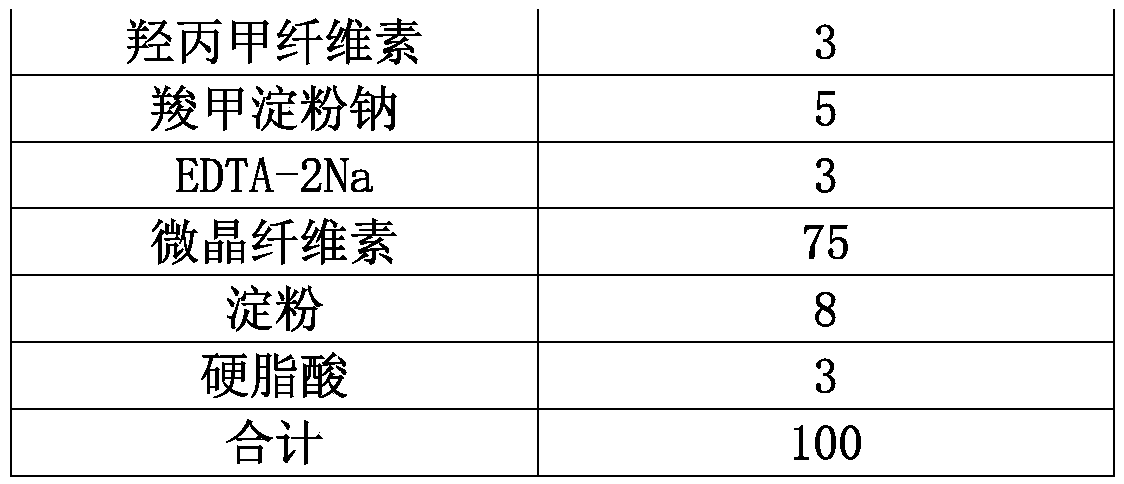
[0030] Embodiment 1: tablet weight 100mg
[0031] prescription:
[0032] Name of raw material
% by weight
1.56
2
8
EDTA-2Na
0.1
50
37.54
0.4
Micropowder silica gel
0.4
total
100
[0033] Process:
[0034] 1. Preparation of binder solution: prepare 5% hypromellose solution, take about 30% of it, add EDTA-2Na and rasagiline mesylate, and stir to dissolve. Take 5% of the prescription amount of microcrystalline fiber and disperse it with water. Mix the binder solution added with the main ingredient and EDTA-2Na with the microcrystalline cellulose suspension, control the temperature of the mixed solution at 30°C to 40°C, stir and disperse for 30 minutes.
[0035] 2. Mix the remaining microcrystalline cellulose, carboxymethyl starch sodium and starch evenly, f...
Embodiment 2
[0037] Embodiment 2: tablet weight 156mg
[0038] (difference from comparative example 1 is that the addition form of microcrystalline fiber of 3% prescription amount is different)
[0039] prescription:
[0040] Name of raw material
percentage%
Rasagiline mesylate
1
2.5
Crospovidone
2
EDTA-2Na
0.5
microcrystalline cellulose
60
precrossified starch
32
1
1
total
100
[0041] Process:
[0042] 1. Preparation of binder solution: prepare 8% hypromellose solution, take about 35% of it, add EDTA-2Na and rasagiline mesylate, and stir to dissolve. Take 3% of the prescription amount of microcrystalline fiber and disperse it with water. Mix the binder solution added with the main ingredient and EDTA-2Na with the microcrystalline cellulose suspension, control the temperature of the mixed solution at 30°C to 40°C, stir and disperse...
Embodiment 3
[0045] Embodiment 3: tablet weight 78mg
[0046] prescription:
[0047] Name of raw material
percentage%
Rasagiline mesylate
2
hypromellose
1.5
3
EDTA-2Na
1.5
microcrystalline cellulose
65
25.2
1
Micropowder silica gel
0.8
total
100
[0048] Process:
[0049] 1. Preparation of binder solution: prepare 10% hypromellose solution, take about 25% of it, add EDTA-2Na and rasagiline mesylate, and stir to dissolve. Get 7% microcrystalline fiber of the prescribed amount and disperse it with water. Mix the binder solution added with the main ingredient and EDTA-2Na with the microcrystalline cellulose suspension, control the temperature of the mixed solution at 30°C to 40°C, stir and disperse for 40 minutes.
[0050] 2. Mix the remaining amount of microcrystalline cellulose, croscarmellose sodium, and lactose evenly, first us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com