Preparation method of lithium/sulfur rechargeable battery electrolyte
A lithium-sulfur secondary battery and electrolyte technology, applied in the field of electrochemistry, can solve problems such as complex process, damage to electrode structure, loss of active material, etc., achieve improved cycle performance and coulombic efficiency, high lithium ion concentration, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
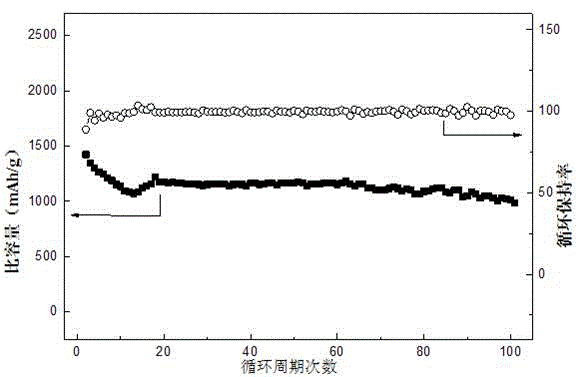
[0023] Put 0.13g of graphene, 0.87g of sublimed sulfur, 0.09g of polyvinylidene fluoride (PVDF), 8mL of N-methylpyrrolidone (NMP) and 20g of grinding beads into a ball ink tank, and ball mill on a ball mill at a speed of 350r / min 4h to obtain positive electrode slurry. The adjusted slurry was uniformly coated on the aluminum foil with a thickness of 200 μm by a film applicator, and placed in a vacuum oven, and baked at 60° C. for 20 hours in a vacuum state. Take 10ml of dimethoxyethane and dioxolane according to the volume ratio, mix them evenly, and weigh 1mol / L LiN(CF 3 SO 2 ) 2 Add dimethoxyethane and dioxolane to the mixed solution, fully stir to dissolve, then add 0.5g elemental sulfur and 0.05g elemental lithium, stir at 60°C for 36h, take the supernatant as the electrolyte, and dry it The pole piece is used as the positive pole piece, and the metal lithium piece is used as the negative pole to assemble a button battery, and a 0.2C rate cycle charge and discharge test...
Embodiment 2
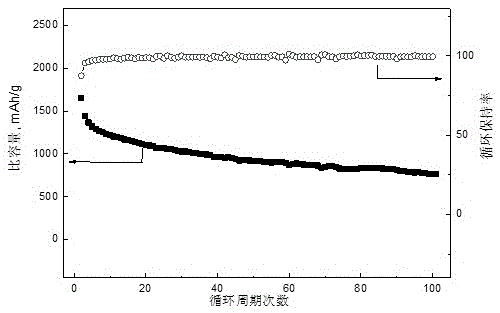
[0027] Put 0.13g of graphene, 0.87g of sublimed sulfur, 0.09g of polyvinylidene fluoride (PVDF), 8mL of N-methylpyrrolidone (NMP) and 20g of grinding beads into a ball ink tank, and ball mill on a ball mill at a speed of 350r / min 4h to obtain positive electrode slurry. The adjusted slurry was uniformly coated on the aluminum foil with a thickness of 200 μm by a film applicator, and placed in a vacuum oven, and baked at 60° C. for 20 hours in a vacuum state. Take 10ml of dimethoxyethane and dioxolane according to the volume ratio, mix them evenly, and weigh 1mol / L LiN(CF 3 SO 2 ) 2 Add dimethoxyethane and dioxolane to the mixed solution, stir and dissolve fully, then add 0.5g elemental sulfur and 0.05g elemental lithium, stir at 35°C for 36h, take the supernatant as the electrolyte, and dry it The pole piece is used as the positive pole piece, and the metal lithium piece is used as the negative pole to assemble a button battery, and a 0.2C rate cycle charge and discharge tes...
Embodiment 3
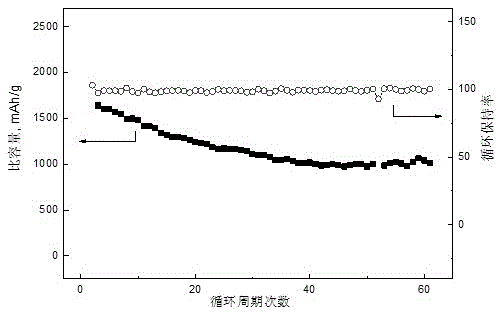
[0029] Put 0.13g of graphene, 0.87g of sublimed sulfur, 0.09g of polyvinylidene fluoride (PVDF), 8mL of N-methylpyrrolidone (NMP) and 20g of grinding beads into a ball ink tank, and ball mill on a ball mill at a speed of 350r / min 4h to obtain positive electrode slurry. The adjusted slurry was uniformly coated on the aluminum foil with a thickness of 200 μm by a film applicator, and placed in a vacuum oven, and baked at 60° C. for 20 hours in a vacuum state. Take 10ml of dimethoxyethane and dioxolane according to the volume ratio, mix them evenly, and weigh 1mol / L LiN(CF 3 SO 2 ) 2 Add dimethoxyethane and dioxolane to the mixed solution, fully stir to dissolve, then add 0.8g elemental sulfur and 0.05g elemental lithium, stir at 35°C for 36h, take the supernatant as the electrolyte, and dry it The pole piece is used as the positive pole piece, and the metal lithium piece is used as the negative pole to assemble a button battery, and a 0.2C rate cycle charge and discharge test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com