Method for analyzing alpha-fluoromethyl acrylate and related substances
A technology for methyl fluoroacrylate and related substances, which is applied in the field of analysis of methyl α-fluoroacrylate and related substances, can solve problems that need to be improved, and achieve good elution and separation effects, strong accuracy, and significant sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
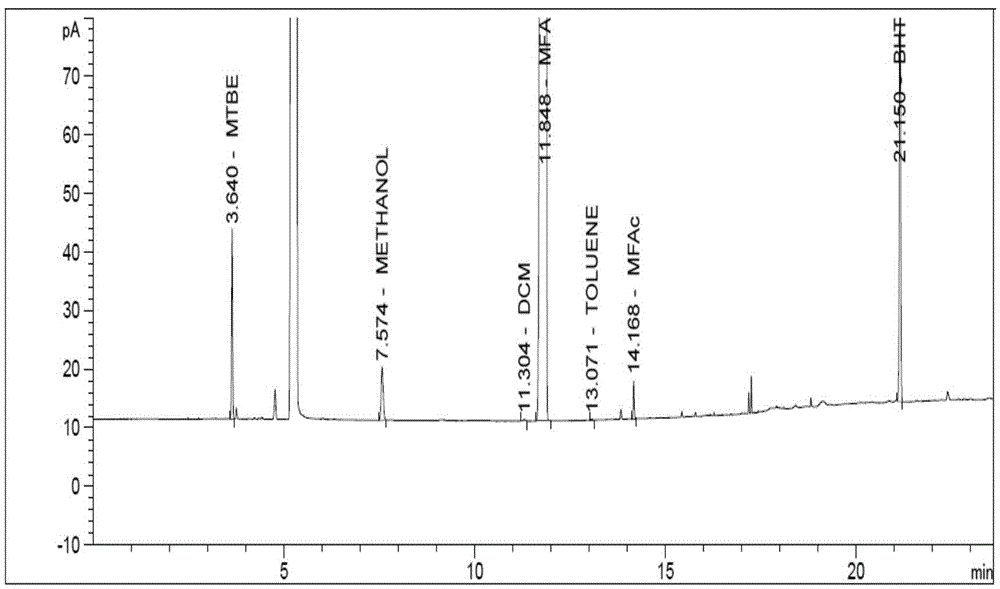
Embodiment 1
[0050] Instrument: Agilent7890A gas chromatograph, FID detector
[0051] Chromatographic column: DB-wax (30m×0.32mm×0.25μm);
[0052] The temperature programming conditions are:
[0053] Heating rate (℃ / min)
[0054] Flow rate: 1.0mL / min
[0055] Detector temperature: 250°C
[0056] Inlet temperature: 240°C
[0057] Injection volume: 1μL
[0058] Split ratio: 50:1
[0059] Diluent: Acetonitrile
[0060] Derivatization reagent: add 60mg trifluoroacetic acid to 2g acetonitrile
[0061] Running time: 23.6min
[0062] Experimental steps:
[0063] BHT reference substance stock solution: about 80mg of BHT reference substance, accurately weighed, put in a 50mL volumetric flask, add diluent to dissolve and dilute to the mark, and shake well.
[0064] Sample and each component reference substance solution: Weigh about 80mg of α-methyl fluoroacrylate reference substance, weigh about 40mg each of MeOH, Toluene, MtBE, DMC, MFAc reference substance, weigh them accurately...
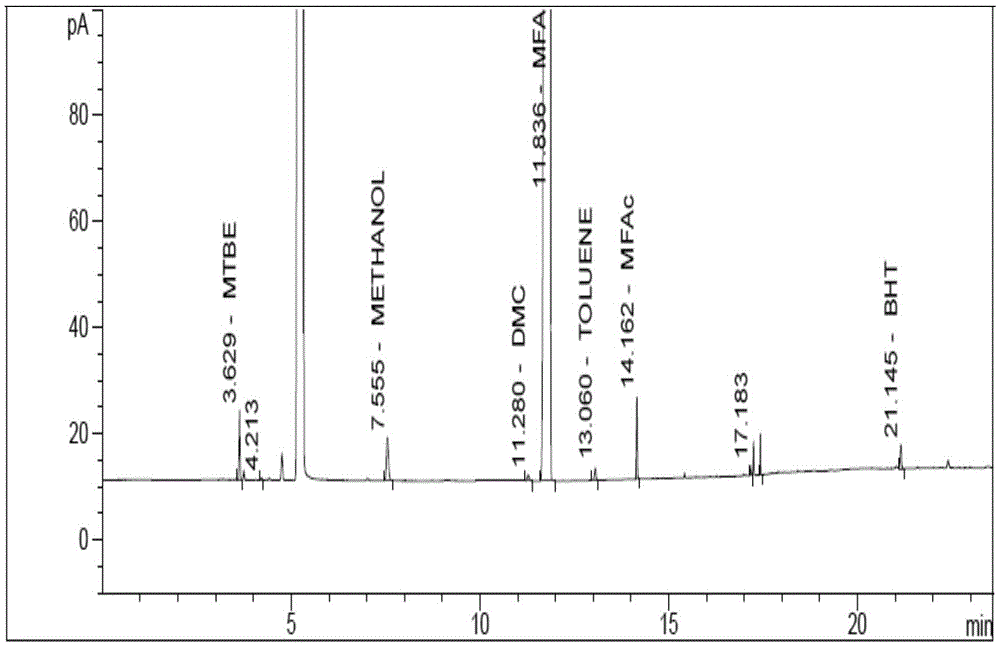
Embodiment 2
[0073] Instrument: Agilent7890A gas chromatograph, FID detector
[0074] Chromatographic column: DB-wax (30m×0.32mm×0.25μm);
[0075] The temperature programming conditions are:
[0076] Heating rate (℃ / min)
[0077] Flow rate: 1.0mL / min
[0078] Detector temperature: 250°C
[0079] Inlet temperature: 240°C
[0080] Injection volume: 1μL
[0081] Split ratio: 50:1
[0082] Diluent: Acetonitrile
[0083] Derivatization reagent: add 60mg trifluoroacetic acid to 2g acetonitrile
[0084] Runtime: 23.6 minutes
[0085] Experimental steps:
[0086] Impurity content reference substance solution: Take about 800mg of MFA reference substance, weigh it accurately, put it into a 10mL volumetric flask with 5mL diluent and 1mL derivatization reagent, add diluent to dissolve and dilute to the mark, shake well (80mg / mL ). Precisely pipette 1mL into a 200mL volumetric flask, add diluent to dilute to the mark, and shake well (0.4mg / mL).
[0087] Sample solution: Take about ...
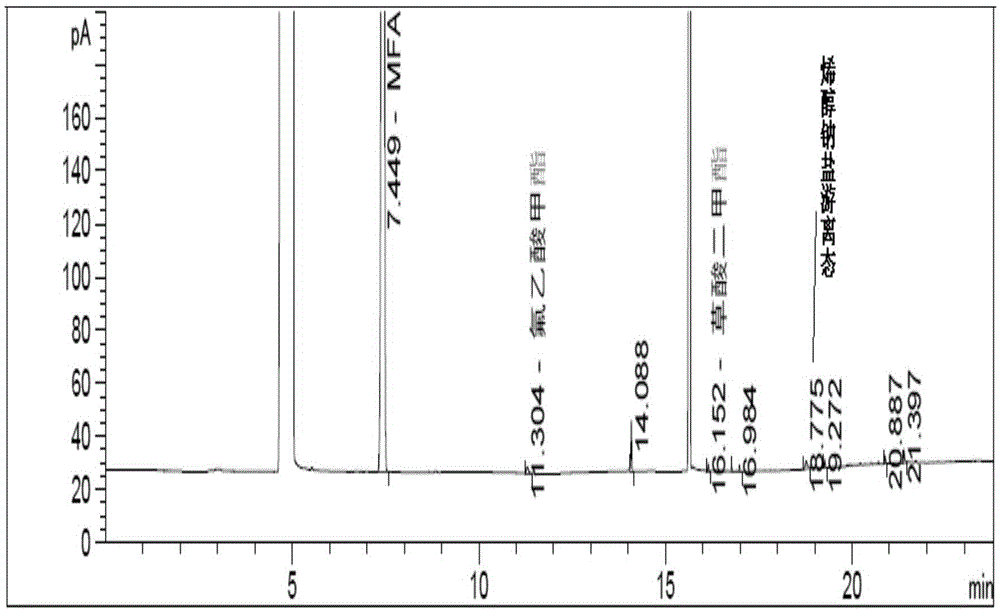
Embodiment 3
[0095] Instrument: Agilent7890A gas chromatograph, FID detector
[0096] Chromatographic column: DB-wax (30m×0.32mm×0.25μm);
[0097] The temperature programming conditions are:
[0098] Heating rate (℃ / min)
temperature(℃)
Hold time (min)
running time (min)
40
8
8
5
60
0
12
25
230
5
23.8
[0099] Flow rate: 1.0mL / min
[0100] Detector temperature: 250°C
[0101] Inlet temperature: 230°C
[0102] Injection volume: 1μL
[0103] Split ratio: 50:1
[0104] Thinner: Acetone
[0105] Derivatization reagent: add 100mg trifluoroacetic acid to 2g acetone
[0106] Running time: 23.8min
[0107] Experimental procedure: Accurately weigh about 200 mg of the MFA reaction solution, add 1 mL of derivatization reagent and 1 mL of diluent, shake well, and filter to obtain the product.
[0108] Conclusion: under this chromatographic condition, the gas chromatogram of gained α-fluoromethyl acrylate react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com