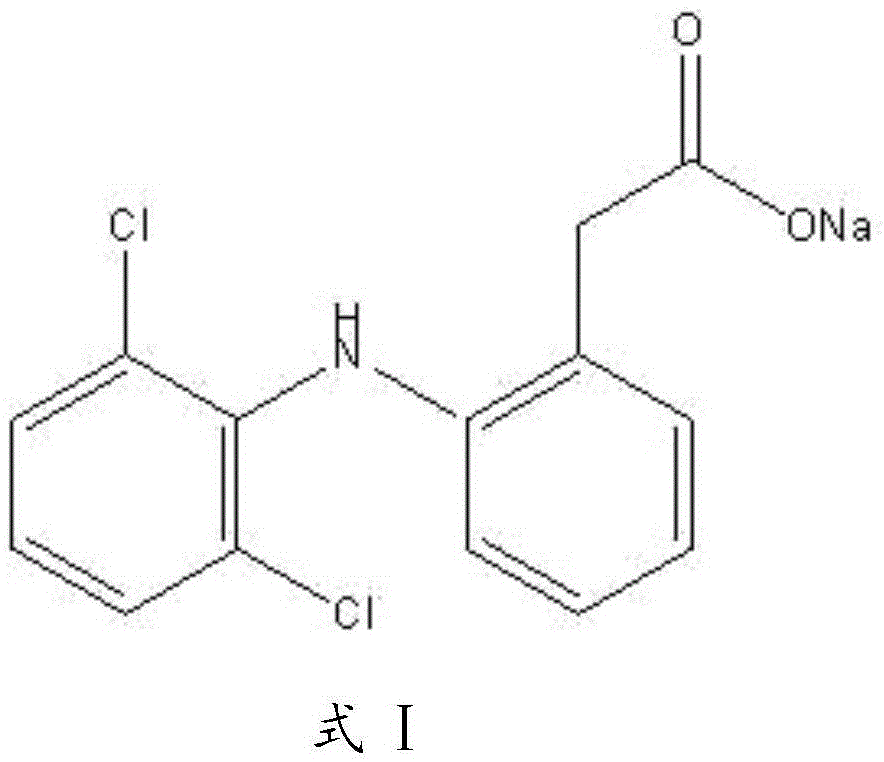
Diclofenac sodium sustained release tablet composition and preparation method thereof
A technology of diclofenac sodium and sustained-release tablets, which is applied in the field of medicine, can solve the problems of difficulty in granulation, flammable and explosive organic solvents, unfavorable production operations, etc., achieves environmental protection, reduces gastric irritation side effects, and has the advantages of health benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
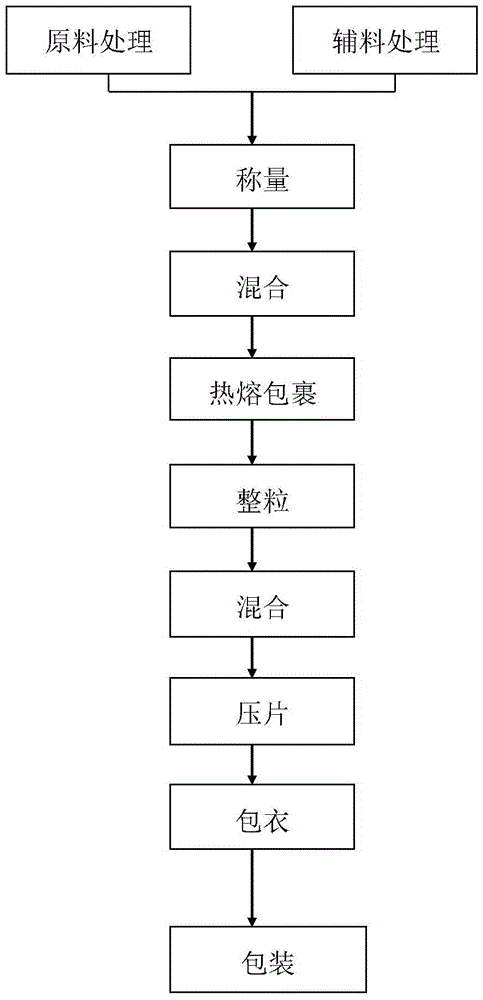
[0063] The preparation process of the above-mentioned diclofenac sodium sustained-release tablets is as follows: take diclofenac sodium, skeleton materials, binders, and fillers and sieve them, put them into a hot-melt wrapping granulator and mix them evenly, then heat up and heat up. The temperature control range is 45-65°C. High-speed shearing and stirring for 5-25 minutes, drug wrapping, granulation, and rounding are completed at one time. After hot-melt wrapping, similar spherical particles are formed, and then sieved through a 20-mesh sieve. After cooling, the granules are granulated through a 18-mesh sieve, and then lubricated. After mixing the preparations for 5 to 15 minutes, tableting and coating were carried out to obtain diclofenac sodium sustained-release tablets.
[0064] In the present invention, by changing the composition ratio of the raw materials and auxiliary materials and the preparation process of the diclofenac sodium sustained-release tablet, a hot-melt w...
Embodiment 1
[0073] formula:
[0074]
[0075] Preparation Process:
[0076] 1. Accurately weigh 1000g of diclofenac sodium, 1060.5g of sodium polystyrene sulfonate, 385.5g of glyceryl monostearate, 385.5g of hydroxypropyl starch, 40g of micropowder silica gel, and 60g of magnesium stearate for later use.
[0077] 2. Put diclofenac sodium, sodium polystyrene sulfonate, hydroxypropyl starch, and micropowder silica gel into a hot-melt wrapping granulator and mix for 5 minutes, then add glyceryl monostearate and continue mixing for 3 minutes, then heat up and control the temperature The range is 55-65°C, high-speed shearing and stirring for 5-15 minutes, after hot-melt wrapping, similar spherical particles are formed, and then sieved through a 20-mesh sieve, let cool, and then pass through a 18-mesh sieve for granulation, and then add lubricant and mix for 10 minutes. Get intermediate.
[0078] 3. For tableting, the weight of 0.1g tablet should be 303mg, and the weight of 75mg tablet sho...
Embodiment 2
[0080] formula:
[0081]
[0082] Preparation Process:
[0083] 1. Accurately weigh 1000g of diclofenac sodium, 640g of polycrylene, 806.5g of cetyl alcohol, 613.5g of sucrose, 50g of micronized silica gel, 40g of magnesium stearate, and 76g of Opadry, and set aside.
[0084] 2. Put diclofenac sodium, polycridine, cetyl alcohol, sucrose, and micropowder silica gel into a hot-melt wrapping granulator and mix for 5 minutes, then heat up, the temperature control range is 58-63°C, high-speed shearing and stirring for 10-25 minutes , to form similar spherical particles after hot-melt wrapping, then sieve through a 20-mesh sieve, let cool, then pass through an 18-mesh sieve for sizing, and then add a lubricant and mix for 10 minutes to obtain an intermediate.
[0085] 3. For tableting, the weight of 0.1g tablet should be 315mg, and the weight of 75mg tablet should be 236.3mg.
[0086] 4. Coating, after coating with Opadry, diclofenac sodium sustained-release tablets were prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com