Pharmaceutical composition of repaglinide and metformin hydrochloride and preparation technology of pharmaceutical composition
A technology of metformin hydrochloride and metformin, which is applied in the directions of drug combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of difficulty in releasing drugs, affecting drug release, and high equipment requirements, and achieving dissolution The effect of consistent curve, product safety and stable particle content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Partial prescription of repaglinide:
[0052]
[0053] Take the material according to the above prescription, mix the active substance, sodium lauryl sulfate and meglumine evenly and pulverize it, then mix it with microcrystalline cellulose and perform dry granulation, then add magnesium stearate and mix to obtain the final mixture .
[0054] The A-D production process is smooth and the product quality is good. The particle results are shown in Table 1 below.
[0055] Table 1 Example 1 experiment related results
[0056]
[0057] Note: The particle content in this article refers to the measured value / theoretical value of the weight percentage of API in the composition in this field. The theoretical value is also called the labeled amount, and it is usually based on the proportion of API in the formulation design.
[0058] It can be seen from the above data that sodium lauryl sulfate can also act as a lubricant during the dry granulation process, and the prepare...
Embodiment 2
[0061]
[0062] Preparation Process:
[0063] Option A
[0064] Part of repaglinide: Mix repaglinide, microcrystalline cellulose, meglumine, and sodium lauryl sulfate evenly, then mix with magnesium stearate, and then carry out dry granulation, and the granules obtained by granulation Diameter 100μm ~ 400μm.
[0065]Metformin hydrochloride part: Metformin hydrochloride and microcrystalline cellulose are evenly mixed, granulated with povidone aqueous solution, and the obtained granules are dried and sized.
[0066] Blending: mix repaglinide granules, metformin hydrochloride granules, and crospovidone granules according to the prescription quantity, then mix with magnesium stearate, and compress into tablets.
[0067] Option B
[0068] Part of repaglinide: Mix repaglinide, microcrystalline cellulose, meglumine, and sodium lauryl sulfate evenly, granulate with purified water, dry, and granulate.
[0069] Metformin hydrochloride part: Metformin hydrochloride and microcrysta...
Embodiment 3
[0082]
[0083] Preparation Process:
[0084] Part of repaglinide: Mix repaglinide, microcrystalline cellulose, meglumine, and sodium lauryl sulfate evenly, then mix with magnesium stearate, and then carry out dry granulation, and the granules obtained by granulation Diameter 100μm ~ 400μm.
[0085] Metformin hydrochloride part: Metformin hydrochloride and microcrystalline cellulose are evenly mixed, granulated with povidone aqueous solution, and the obtained granules are dried and sized.
[0086] Blending: mix repaglinide granules, metformin hydrochloride granules, and crospovidone granules according to the prescription quantity, then mix with magnesium stearate, and compress into tablets.
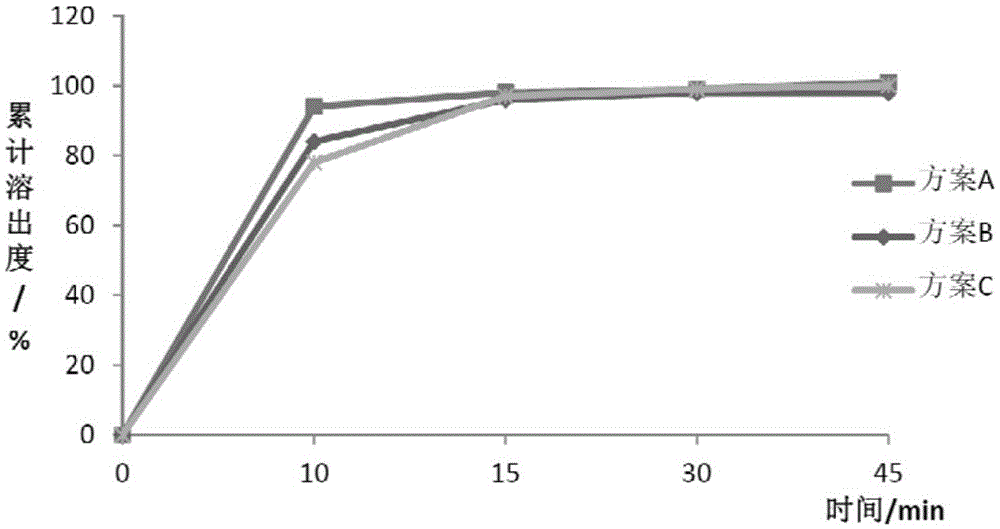
[0087] Table 4 Dissolution data of repaglinide
[0088]
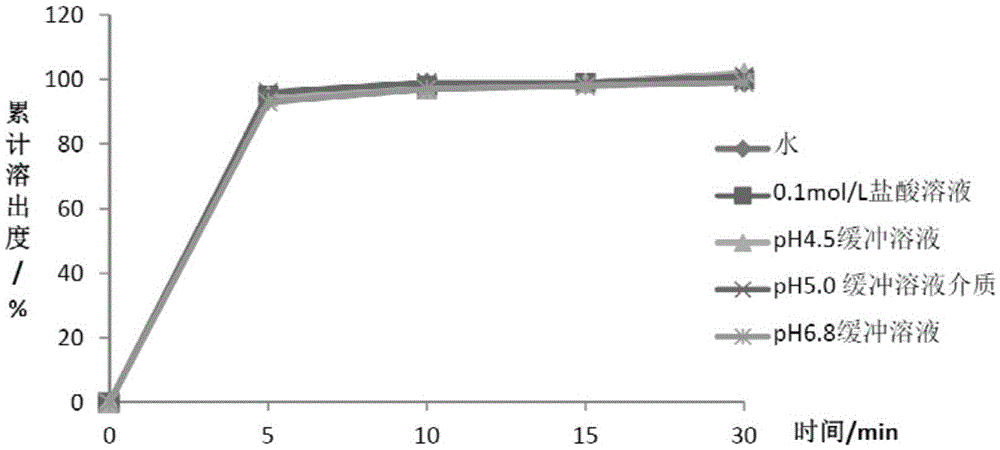
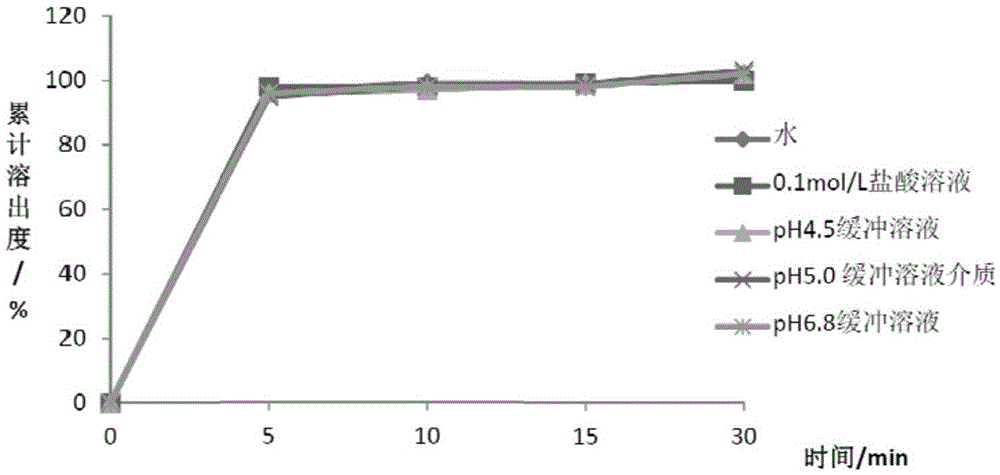
[0089] Table 5 Metformin Hydrochloride Dissolution Data
[0090]
[0091] It can be seen from the above data that the two active substances can achieve rapid dissolution in different dissolution media, and the dissoluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com