Orixine hydrochloride as well as preparation method and medical application thereof
A technology of fushenzine hydrochloride and hydrochloric acid gas, which is applied in the field of fushenzine hydrochloride and its preparation, and can solve problems such as the difficulty of large-scale production of fushenzine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
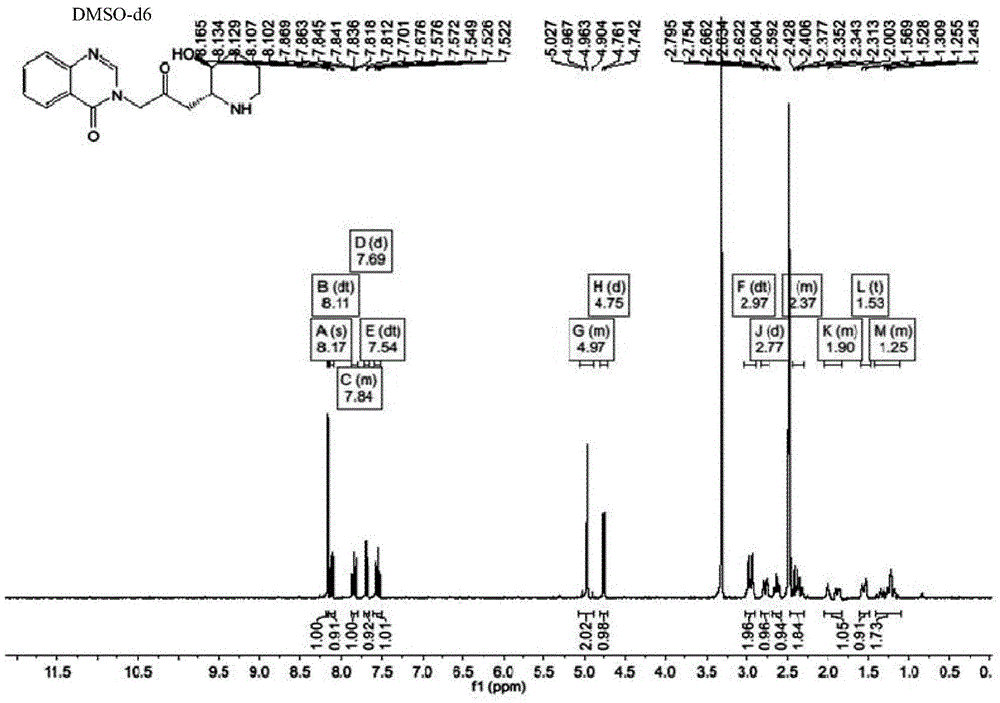
[0033] The preparation of embodiment 1 halosine hydrochloride
[0034] Take the coarse powder of Changshan medicinal materials, extract by 70% ethanol heat reflux, recover the solvent from the extract until it has no alcohol smell, first add concentrated hydrochloric acid to adjust the pH value to about 2.5, then add chloroform to extract to remove acidic impurities, add concentrated ammonia water to the raffinate phase to adjust the pH value to about 9.0, then add chloroform for extraction, and recover the solvent from the chloroform extract to obtain the total alkaloids of Changshan.
[0035] Add absolute ethanol to the obtained total alkaloids of Changshan to dissolve and filter to obtain a solution, and directly pass hydrochloric acid gas into the absolute ethanol solution of total alkaloids of Changshan for 4 hours to obtain a precipitate. The precipitate was recrystallized three times with absolute ethanol to obtain halosine hydrochloride. Detected by HPLC, its content ...
Embodiment 2
[0039] Embodiment 2 studies on the stability of halosine and halosine hydrochloride
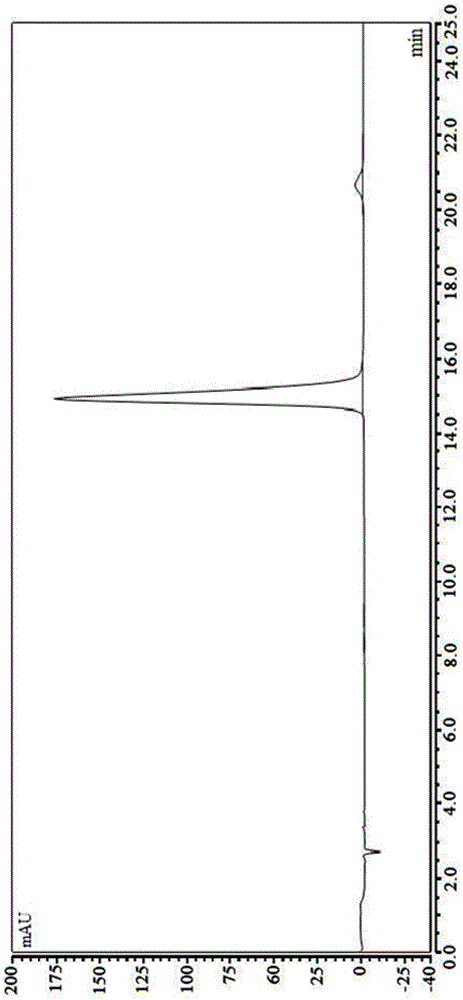
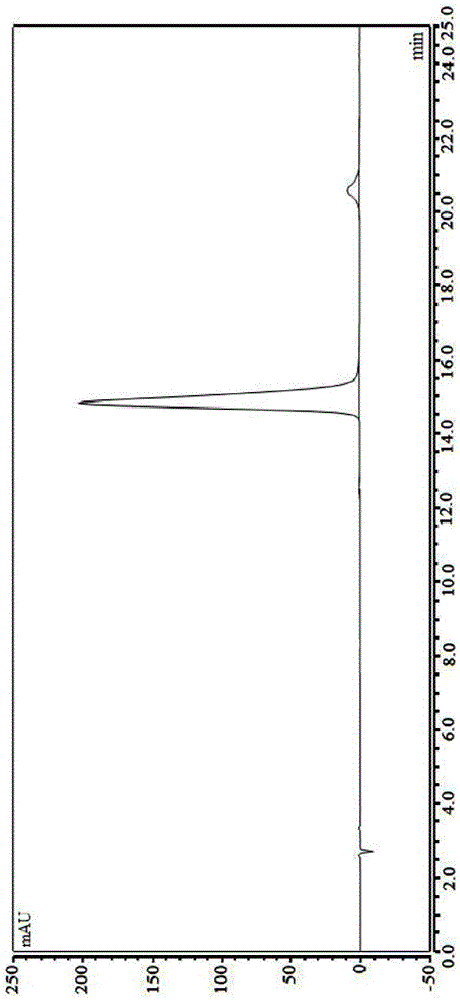
[0040] The stability of halosine and halosine hydrochloride was determined by HPLC.
[0041] The chromatographic conditions are as follows: Agilent1200 high performance liquid chromatography; chromatographic column: YWG-C 18 (4.6mm×250mm, 10μm); mobile phase: acetonitrile-water-triethylamine-glacial acetic acid (9:91:0.35:0.75); detection wavelength: 225nm; column temperature: 30°C; flow rate: 1mL / min. Sample concentration: 1.0 mg / mL; injection volume: 10 μL.
[0042] Preparation of the reference substance solution: Precisely weigh the reference substance of Changshan alkali (references: Zhang Ya, Li Chun, Lei Guolian. Research on the chemical constituents of Changshan. Chinese Journal of Experimental Formulas, 2010,16(5):77-79. preparation) and the as-prepared hirshenine hydrochloride (put into a decompression desiccator to dry for 24 hours after the hirsutine hydrochloride is prepared) ea...
Embodiment 3
[0045] Example 3 Research on the Water Solubility of Hemosine and Hemosine Hydrochloride
[0046] Grind the hematine hydrochloride prepared above into a fine powder, accurately weigh 10 mg at room temperature, add 50 μL of deionized water, shake vigorously for 30 seconds every 5 minutes, and there will be no visible solute particles within 30 minutes , which is completely dissolved. According to the regulations on solubility in the 2015 edition of "Chinese Pharmacopoeia", hirsutine hydrochloride is easily soluble in water [easy soluble: means that 1 g (mL) of solute can be dissolved in 1 to less than 10 mL of solvent].
[0047] Grind firdosine into a fine powder, accurately weigh 10 mg at room temperature, gradually add deionized water, shake vigorously for 30 seconds every 5 minutes, until nearly 100 mL of water is added, firdosine is completely dissolved, so firdosine is in water The solubility is very slightly soluble [very slightly soluble: means that 1g of solute can be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com