Protective antigen protein of porcine toxigenic pasteurella multocida as well as application and vaccine of protective antigen protein
A technology of protective antigen and Pasteurella, which is applied in the field of immunology, can solve the problems of low production capacity, unfavorable scale-up production, unsatisfactory protein expression, and increased production cost of the production line, so as to increase production practicability and good immunity Originality, conducive to the effect of production amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Construction of embodiment 1 recombinant strain
[0033] 1.1 Construction of expression engineering bacteria
[0034] According to literature research: the full length of PMT is toxic, and PMT-N is more immunogenic than the C-terminal of PMT. Afterwards, we constructed 3 strains expressing the full-length of PMT, PMT-N (PMT N-terminal 1~ 520 fragment), PMT-C protein (PMT 520-1295 fragment), PET-PMT full-length (BL21), PET-PMT-N (BL21), PET-PMT-C (BL21) expression engineering bacteria .
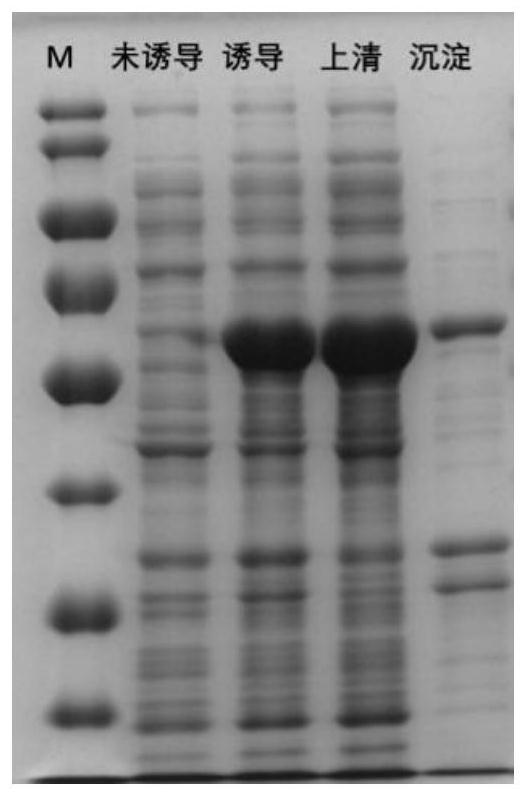
[0035] 1.2 Full-length PMT, PMT-N, PMT-C protein expression, verification and purification quantification
[0036]Express engineering bacteria PET-PMT full-length (BL21), PET-PMT-N (BL21), PET-PMT-C (BL21) were streaked on LB (corresponding to resistance) solid plates and cultured at 37°C for 16 hours, and single The colonies were inoculated in 5ml LB liquid medium containing 100μg / ml corresponding resistance, cultured on a shaker (150r / min) at 37°C for 16h, and then inoculated at 1%...
Embodiment 2
[0038] Example 2. Vaccine Preparation
[0039] 2.1 Preparation of PMT full-length vaccine, PMT-N vaccine, PMT-C vaccine, PMT-N+PMT-C vaccine
[0040] Take a certain amount of PMT full-length protein (1), PMT-N protein (2), PMT-C protein (3) and PMT-N+PMT-C protein (4) prepared in Example 1 and add a certain amount of Bowers' protein Whole bacteria inactivated liquid, add a certain proportion of water-based adjuvant, stir evenly with a magnetic stirrer, and then sub-package. After passing the inspection, it is the porcine atrophic rhinitis subunit vaccine, wherein the antigen content in the vaccine is 50-200 μg / ml, The ratio of antigen and adjuvant in the vaccine is as follows:
[0041] Table 1 Composition of vaccines 1, 2, 3, 4
[0042]
[0043]
[0044] Note: "-" means no addition.
Embodiment 3
[0045] Embodiment 3. Vaccine sterility test
[0046] The sterility test was performed on the above three subunit vaccines in accordance with the appendix of the current "Chinese Veterinary Pharmacopoeia". The results were sterile growth.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com