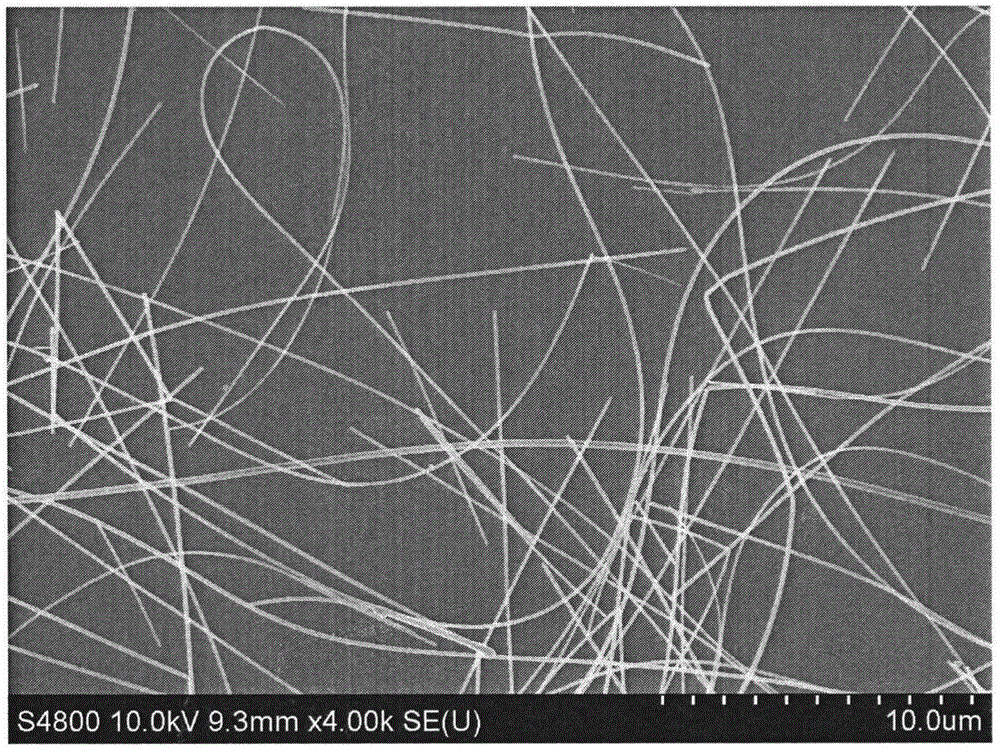
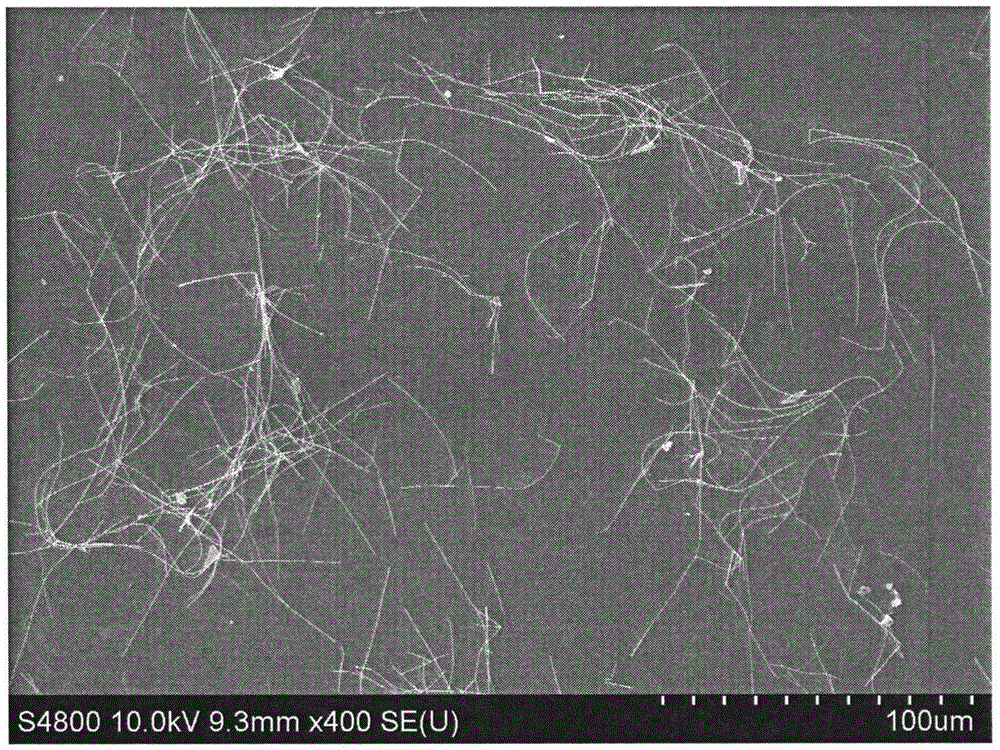
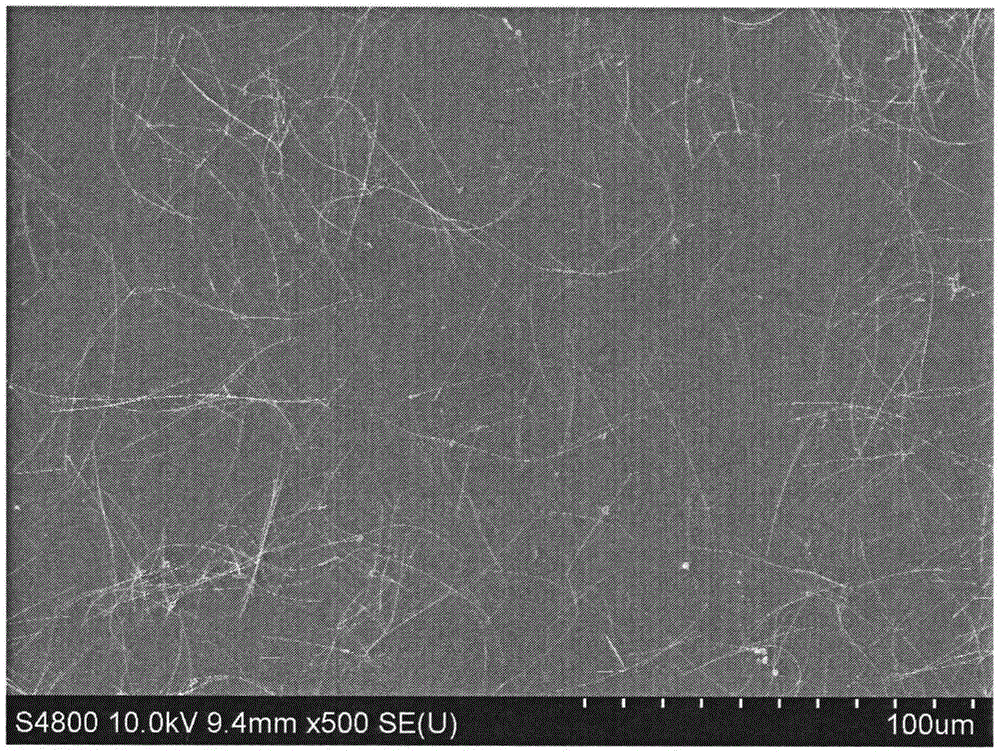
Method for preparing silver nanowires
A technology of silver nanowires and silver salts is applied in the field of preparing silver nanowires, which can solve the problems of high template requirements and limited number of nanowires, and achieve the effects of improving light transmittance, facilitating enlarged production, and reducing silver particles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Fill a 250mL three-necked reaction flask with a condensation reflux device with nitrogen, and preheat in an oil bath at 140°C; under nitrogen protection, add 0.5g of polyvinylpyrrolidone K60 to a 250mL conical flask, add 25mL of ethylene glycol and stir for 1 1 hour until fully dissolved; then take 0.25g of silver nitrate and add it to the above-mentioned Erlenmeyer flask, stir for 1 hour until fully dissolved; add 1.5g of ethylene glycol solution of ferric chloride with a molar concentration of 1 μM, and stir for 2 minutes; The above-mentioned mixed solution in the Erlenmeyer flask was poured into a three-necked reaction flask preheated before, and reacted for 5 hours.
[0028] Cool the reaction solution to room temperature, transfer the obtained mother solution to a centrifuge tube, add acetone with a volume 9 times the volume of the mother solution, shake it vigorously and settle naturally for 20 minutes, remove the supernatant, keep the bottom precipitate, repeat thi...
Embodiment 2
[0030] Fill a 250mL three-necked reaction flask with a condensation reflux device with nitrogen, and preheat in an oil bath at 140°C; under nitrogen protection, add 0.5g of polyvinylpyrrolidone K30 to a 250mL conical flask, add 25mL of ethylene glycol and stir for 1 1 hour until fully dissolved; then take 0.25g of silver nitrate and add it to the above-mentioned Erlenmeyer flask, stir for 1 hour until fully dissolved; add 1.5g of ethylene glycol solution of ferric chloride with a molar concentration of 1 μM, and stir for 2 minutes; The above-mentioned mixed solution in the Erlenmeyer flask was poured into a three-necked reaction flask preheated before, and reacted for 5 hours.
[0031] Cool the reaction solution to room temperature, transfer the obtained mother solution to a centrifuge tube, add acetone with a volume 9 times the volume of the mother solution, shake it vigorously and settle naturally for 20 minutes, remove the supernatant, keep the bottom precipitate, repeat thi...
Embodiment 3
[0033] Fill a 250mL three-necked reaction flask with a condensation reflux device with nitrogen, and preheat it in an oil bath at 140°C; under nitrogen protection, add 0.5g of polyvinylpyrrolidone K90 to a 250mL Erlenmeyer flask, add 25mL of ethylene glycol and stir for 1 1 hour until fully dissolved; then take 0.25g of silver nitrate and add it to the above-mentioned Erlenmeyer flask, stir for 1 hour until fully dissolved; add 1.5g of ethylene glycol solution of ferric chloride with a molar concentration of 1 μM, and stir for 2 minutes; The above-mentioned mixed solution in the Erlenmeyer flask was poured into a three-necked reaction flask preheated before, and reacted for 5 hours.
[0034]Cool the reaction solution to room temperature, transfer the obtained mother solution to a centrifuge tube, add acetone with a volume 9 times the volume of the mother solution, shake it vigorously and settle naturally for 20 minutes, remove the supernatant, keep the bottom precipitate, repea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com