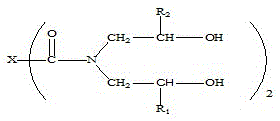
Beta-hydroxyalkylamide and preparation method thereof
A technology of hydroxyalkylamide and alkyl, which is applied in the field of curing agent and its preparation, can solve the problems of harsh synthesis conditions and low yield, and achieve the effects of low production cost, simple synthesis and strong reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
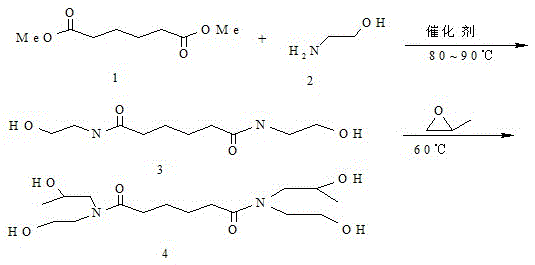
[0034] Preparation of N,N'-bis(β-hydroxyethyl)adipamide
[0035] Place 0.5mol dimethyl adipate and 1mol ethanolamine in a four-necked flask, then add 0.6g potassium hydroxide as a catalyst, under N 2 Under protection, the reaction was heated and refluxed, and the reaction temperature was controlled at about 90°C with a temperature-controlled water bath until no methanol distilled out, and the reaction was terminated. The obtained product crystallized immediately after standing at room temperature to obtain a pale yellow solid. The light yellow solid was recrystallized with methanol and acetone to obtain a white solid, which was placed in a vacuum oven and dried.
Embodiment 2
[0037] Preparation of N,N'-bis(β-hydroxyethyl)-N,N'-bis(β-hydroxypropyl)adipamide 0.88gN,N'-bis(β-hydroxyethyl)adipamide Dissolve in 3.7g dimethylformamide, slowly add 0.44g propylene oxide dropwise at 60°C, after the dropwise addition is completed, heat up and reflux for 2 hours; cool down to room temperature, wash the product with 20% NaOH, distill the solvent, white The residue is refined with ethanol to obtain the target product with a content greater than 99%.
Embodiment 3
[0039] Preparation of N,N'-bis(β-hydroxyethyl)adipamide Place 0.5mol dimethyl adipate and 1mol ethanolamine in a four-necked flask, then add 2.6g n-butyl titanate as a catalyst, N 2 Under protection, the reaction was heated and refluxed, and the reaction temperature was controlled at about 90°C with a temperature-controlled water bath until no methanol distilled out, and the reaction was terminated. The obtained product crystallized immediately after standing at room temperature to obtain a white transparent solid. The white solid was dried in a vacuum oven.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com