Preparation method of 2-butyl sulfoxide-1,4-naphthoquinone
A naphthoquinone and sulfhydryl technology, applied in the field of preparation of 1,4-naphthoquinone, can solve the problems of low reaction yield, reduced anticancer activity, harsh reaction conditions, etc., and achieves simple synthesis route, obvious anticancer effect, The effect of low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The following steps are used to prepare 2-butylsulfoxide-1,4-naphthoquinone:
[0026] (1) Synthesis of 2-butylmercapto-1,4-naphthoquinone
[0027] In a 100ml reaction bottle, add 158.15mg (1mmol) of 1,4-naphthoquinone and 30ml of methanol, mix well, add 166μl (1.5mmol) of 1-butanethiol, and react at room temperature for 4 hours, then add dichrome to the mixture Sodium acid 59.6mg (0.2mmol) and concentrated sulfuric acid 40.8μl (0.75mmol), the reaction is completed after 5-10 minutes. Extracted with dichloromethane and saturated saline, dried with an appropriate amount of anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain a crude product, which was prepared by TLC to obtain 2-butylmercapto-1,4-naphthoquinone;
[0028] (2) Synthesis of 2-butylsulfoxide-1,4-naphthoquinone (BSNQ)
[0029] In a 50ml reaction bottle, add 246.32mg (1mmol) of the above-mentioned product 2-butylmercapto-1,4-naphthoquinone and 20ml of chloroform, slowly add 276.1mg (1.2mm...
experiment example
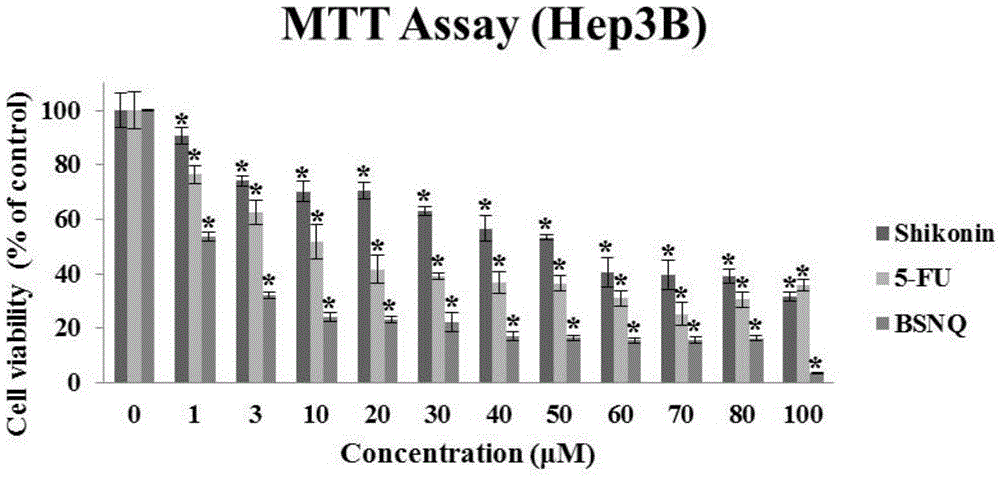
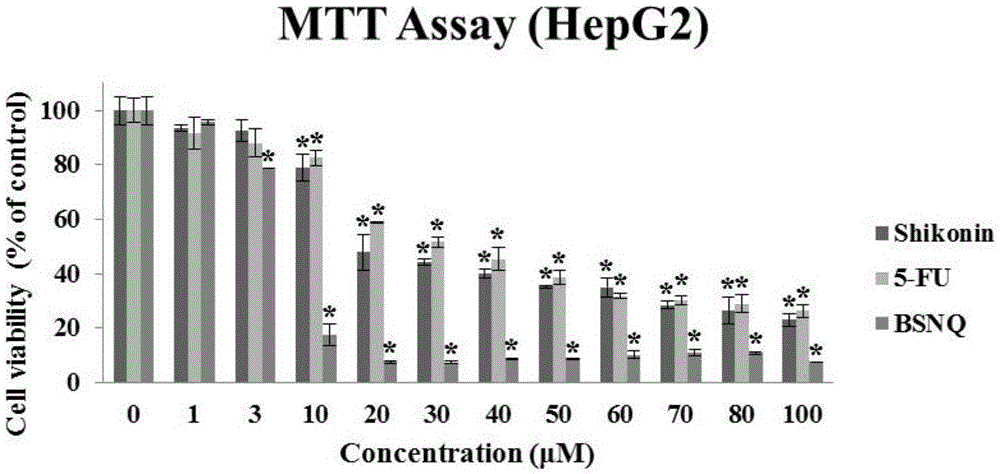
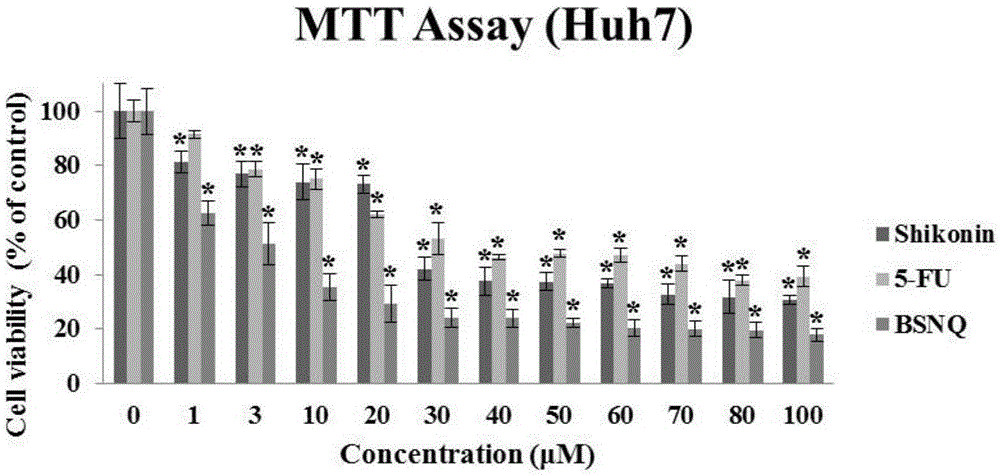
[0031] 1. The killing effect of BSNQ on cancer cells
[0032] Experiment method: (MTT experiment)
[0033] ① Cell inoculation: use culture medium containing 10% fetal calf serum to prepare a single cell suspension, inoculate 10,000 cells per well into a 96-well plate, and the volume of each well is 200 μl;
[0034] ② Culture cells: 5% CO 2 , incubate at 37°C for 24 hours until the cell monolayer covers the bottom of the well;
[0035] ③ Serum starvation: Change the culture medium (culture medium containing 1% FBS) 2 hours before adding the drug;
[0036] ④ Drug treatment: The prepared BSNQ was treated with final concentrations of 0, 1, 3, 10, 30, 40, 50, 60, 70, 80, and 100 μM to treat human liver cancer Hep3B, HepG2, and Huh7 cells for 24 hours;
[0037] ⑤ Color reaction: Add 20 μl of MTT solution (5 mg / ml, prepared in PBS, pH 7.4) to each well. After continuing to incubate for 2-4 hours, carefully aspirate and discard the culture supernatant in the well, carefully wash t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com