Metal organic complex containing thiophene functional group and preparing method and application of metal organic complex
A functional group, metal-organic technology, which is applied in the field of porous metal-organic complexes and their preparation, can solve problems such as poisoning, impaired liver and kidney functions and immunity, and anemia, and achieves the effect of a simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
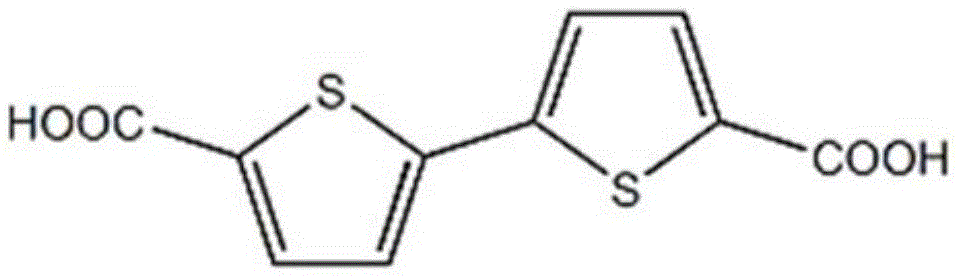
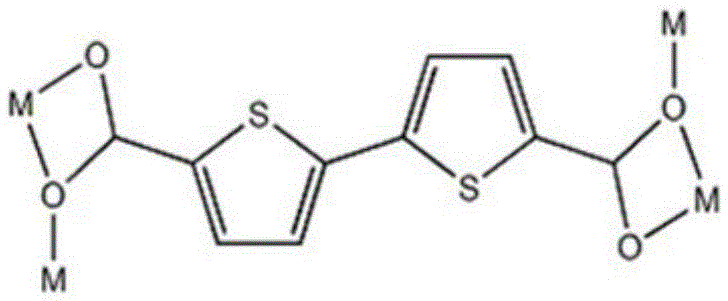
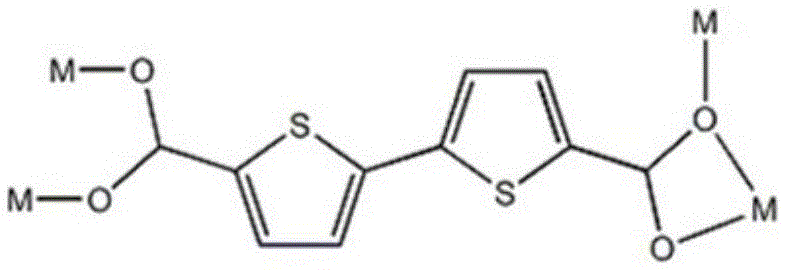
[0028] Embodiment 1 contains the organometallic complex of thiophene functional group
[0029] H 2 btdc (0.005g, 0.02mmol) and Cd(NO 3 ) 2 ·6H 2 O (0.015g, 0.05mmol) was added into a high-temperature-resistant screw-top glass bottle with a volume of 5mL, 0.75mL of DMF was added, stirred with a magnetic stirrer to dissolve it, and then 0.75ml of C 2 h 5 OH, stir for 15 minutes until the metal salt is completely dissolved and mixed evenly with the ligand, seal the glass bottle and put it in an oven at 75°C, heat at this temperature for 2 days, at 5°C·h -1 The cooling rate was slowly cooled to room temperature, and yellow blocky crystals were generated. They were washed with N,N-dimethylformamide, filtered, and dried to obtain the target product, which was a metal-organic complex containing thiophene functional groups. The yield was 58%.
[0030] Elemental analysis calculated value (%): C30.64, H1.97; measured value (%): C30.70, H2.01.
[0031] Infrared spectrum (KBr, cm ...
Embodiment 2
[0034] Example 2 Fluorescence properties and selective recognition of metal ions of a metal-organic complex containing thiophene functional groups
[0035] Method: A metal-organic complex containing thiophene functional group prepared in Example 1 was used as a fluorescent sensor to selectively recognize metal ions.
[0036] 1) The metal-organic complexes containing thiophene functional groups of the present invention are subjected to solid and liquid fluorescence luminescence tests at room temperature.
[0037] Liquid sample configuration: Take a certain amount of metal-organic complex crystals containing thiophene functional groups, grind to uniform powder, then dissolve in methanol solution, and configure to a concentration of 1×10 -4 Methanol suspension of M.
[0038] The results of solid-state fluorescence analysis show that the metal-organic complex containing thiophene functional groups exhibits a strong emission band at about 468 nm under excitation at a wavelength of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com