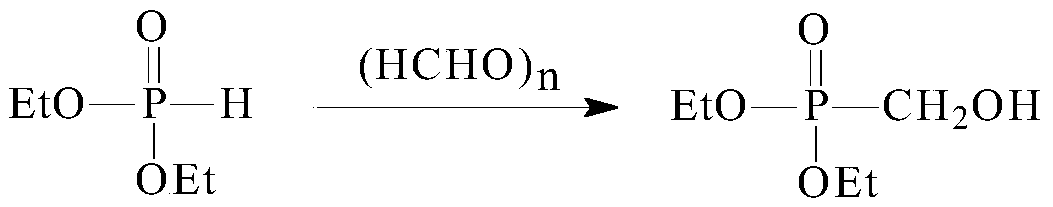Diethyl p-toluenesulfonyloxy methylphosphonate synthesis method
A technology of diethyl toluenesulfonyloxymethylphosphonate and diethyl hydroxymethylphosphonate is applied in the field of synthesis of diethyl p-toluenesulfonyloxymethylphosphonate, which can solve the problem of increasing cost, difficulty in Avoid problems such as excessive water content of diethyl hydroxymethyl phosphonate, and achieve the effect of saving energy consumption, reducing post-processing procedures, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic method of described p-toluenesulfonyloxymethylphosphonic acid diethyl ester, comprises the steps:
[0033] (1) Add 30.0g of diethyl phosphite, 30mL of isopropanol, 1.0g of potassium carbonate and 7.8g of paraformaldehyde to a 250mL three-necked flask in sequence, raise the temperature to 84°C for reaction, and detect the reaction by GC after the completion of the reaction (raw material diphosphite The residue of ethyl ester is less than 0.2%), potassium carbonate is filtered out, the mother liquor is evaporated to recover isopropanol, and the distillation residue is diethyl hydroxymethylphosphonate;
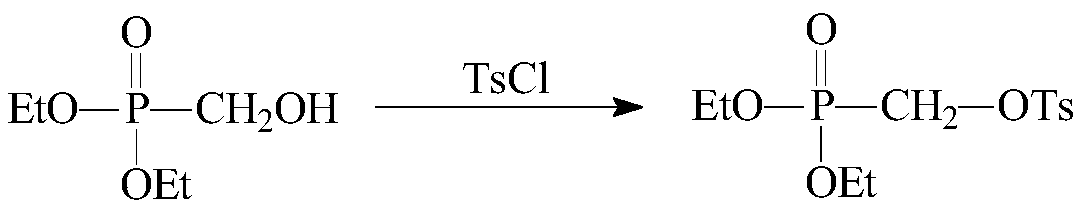
[0034] (2) Cool down the diethyl hydroxymethylphosphonate prepared in step (1) to 0°C, add 60mL of dichloromethane and 37.3g of p-toluenesulfonyl chloride, and then add 26.4g of triethylamine dropwise. Continue to stir for 2h, heat up to 26°C and stir, TLC monitors the reaction (stop the reaction when there is no p-toluenesulfonyl chloride remaining), after t...
Embodiment 2
[0036] The synthetic method of described p-toluenesulfonyloxymethylphosphonic acid diethyl ester, comprises the steps:
[0037] (1) Add 30.0g of diethyl phosphite, 30mL of isopropanol, 1.0g of potassium carbonate and 7.8g of paraformaldehyde into a 250mL three-neck flask, heat up to 86°C for reaction, and after the reaction is complete by GC detection (raw material diethyl phosphite Esters residue is lower than 0.2%), potassium carbonate is filtered out, the mother liquor is evaporated to recover isopropanol and used mechanically, and the distillation residue is diethyl hydroxymethylphosphonate;
[0038] (2) Cool down the diethyl hydroxymethylphosphonate prepared in step (1) to 0°C, add 60mL of dichloromethane and 37.3g of p-toluenesulfonyl chloride, and then add 26.4g of triethylamine dropwise. Continue to stir for 2h, heat up to 26°C and stir, TLC monitors the reaction (stop the reaction when there is no p-toluenesulfonyl chloride remaining), after the reaction is complete, ...
Embodiment 3
[0040] The synthetic method of described p-toluenesulfonyloxymethylphosphonic acid diethyl ester, comprises the steps:
[0041] (1) Add 30.0g of diethyl phosphite, 30mL of dichloromethane, 1.0g of potassium carbonate and 8.0g of paraformaldehyde to a 250mL three-necked flask in sequence, raise the temperature to 82°C for reaction, and GC detects that the reaction is complete (the raw material phosphite di Ethyl residue is less than 0.2%), potassium carbonate is filtered out, the mother liquor is distilled out of dichloromethane for recycling, and the distillation residue is diethyl hydroxymethylphosphonate;
[0042] (2) Cool the diethyl hydroxymethylphosphonate prepared in step (1) to -1°C, add 60mL of dichloromethane and 37.3g of p-toluenesulfonyl chloride, then add 25.3g of triethylamine dropwise. Then continue to stir for 1.5h, heat up to 26°C and stir, TLC monitors the reaction (when there is no residue of p-toluenesulfonyl chloride, stop the reaction), after the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com