23-hydroxybetulinic acid fluorescent probe and its preparation method and use in cellular localization and uptake
A fluorescent probe, the technology of betulinic acid, which is applied in the detection of the application of 23-hydroxy betulinic acid in the localization and uptake of target cells, the preparation of a fluorescent probe of 23-hydroxy betulinic acid, a natural product of anti-tumor activity, in the field of fluorescent probes, It can solve the problems that the molecular mechanism of action remains to be explored, the anti-tumor molecular mechanism of 23-HBA has not been fully elucidated, and the cellular target and mode of action are unclear, and achieve the effect of good effect, mild conditions and anti-tumor effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 23-HBA fluorescent probe
[0042] Compound a (2g, 7.7mmol), N-hydroxysuccinimide (1.3g, 11.5mmol) and EDCI (1.8g, 9.2mmol) were dissolved in dichloromethane (20.0mL), stirred at room temperature for 4h, concentrated Add water, extract with dichloromethane (30mL×3), combine the organic layers, wash with water, wash with saturated brine, anhydrous Na 2 SO 4 After drying and concentration, column chromatography (CH 2 Cl 2 / CH 3 OH 100:1, v / v) yielded the target compound b (2 g, 72.9%). 1 H-NMR (CDCl 3 , 300MHz) δ: ppm1.26 (6H, t, J = 7.1Hz), 2.90 (4H, s), 3.48 (4H, q, J = 7.1Hz), 6.45 (1H, d, J = 1.9Hz, Ar -H), 6.65 (1H, dd, J=9.0Hz, J=2.2Hz, Ar-H), 7.38 (1H, d, J=9.0Hz, Ar-H), 8.59 (1H, s, Ar-H ).
[0043] Dissolve compound b (700mg, 1.95mmol), N-Boc-ethylenediamine (0.4mL) and DIPEA (1.4mL) in dichloromethane (20.0mL), stir at room temperature for 3h, add water after concentration, dichloromethane Extract (30mL×3), combine the organic layers, wash w...
Embodiment 2
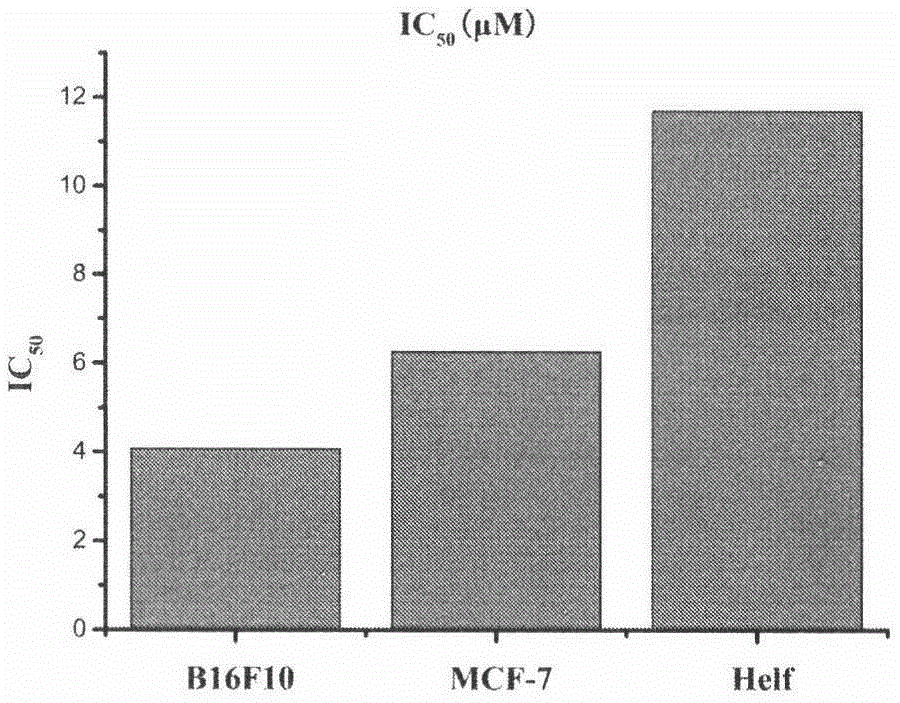
[0049] Anti-tumor Proliferation Activity of 23-HBA Fluorescent Probe
[0050] The tumor cells in the logarithmic growth phase were digested, counted, and prepared at a concentration of 5×10 4 cells / mL of cell suspension, 100 μL of cell suspension was added to each well of a 96-well plate (5×10 3 cells). Place the 96-well plate at 37°C, 5% CO 2 After culturing in the incubator for 24 hours, the drug was diluted to the desired concentration with complete medium, and 100 μL of the corresponding drug-containing medium was added to each well. Place the 96-well plate at 37°C, 5% CO 2 After culturing in the incubator for 72 hours, stain the 96-well plate with MTT; add 20 μL MTT (5 mg / mL) to each well, and continue culturing in the incubator for 4 hours; discard the medium, add 150 μL DMSO to each well to dissolve, and shake gently for 10 minutes. Mix well; λ=490nm, read the OD value of each well with a microplate reader, and calculate the inhibition rate. Experimental results (s...
Embodiment 3
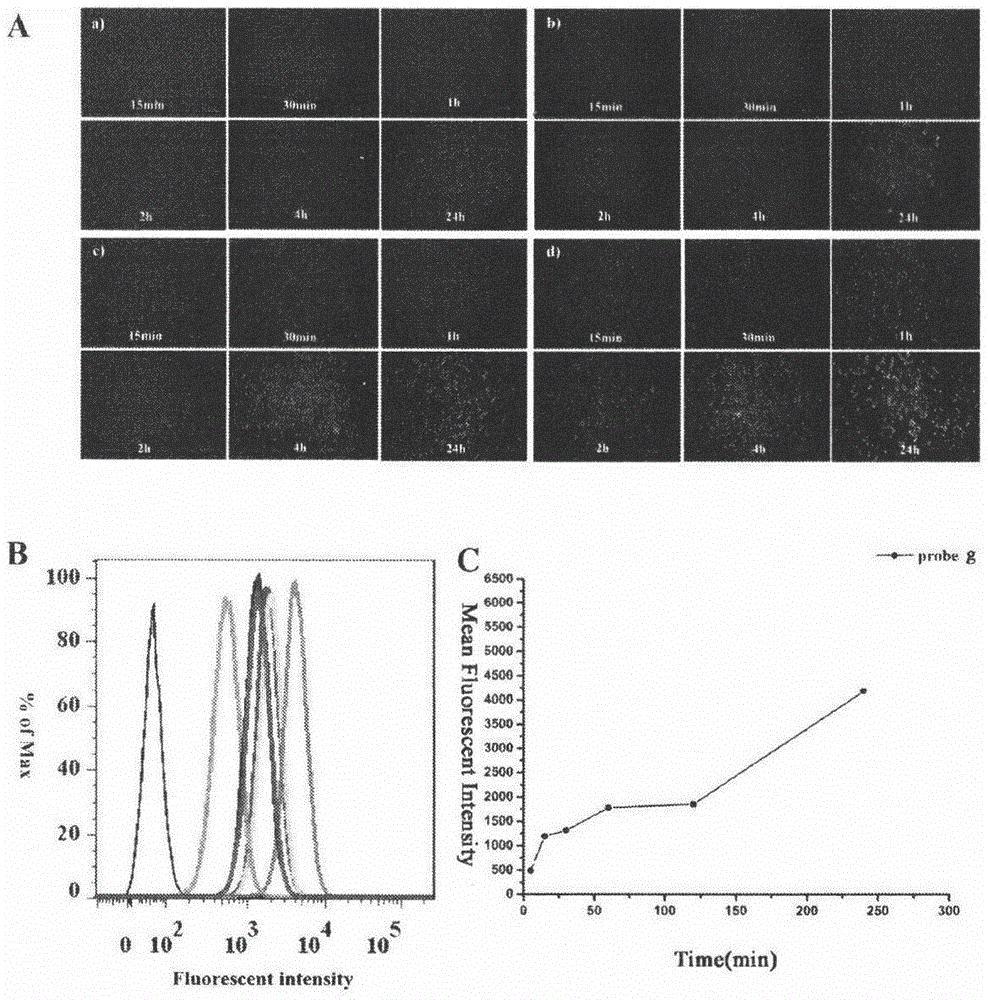
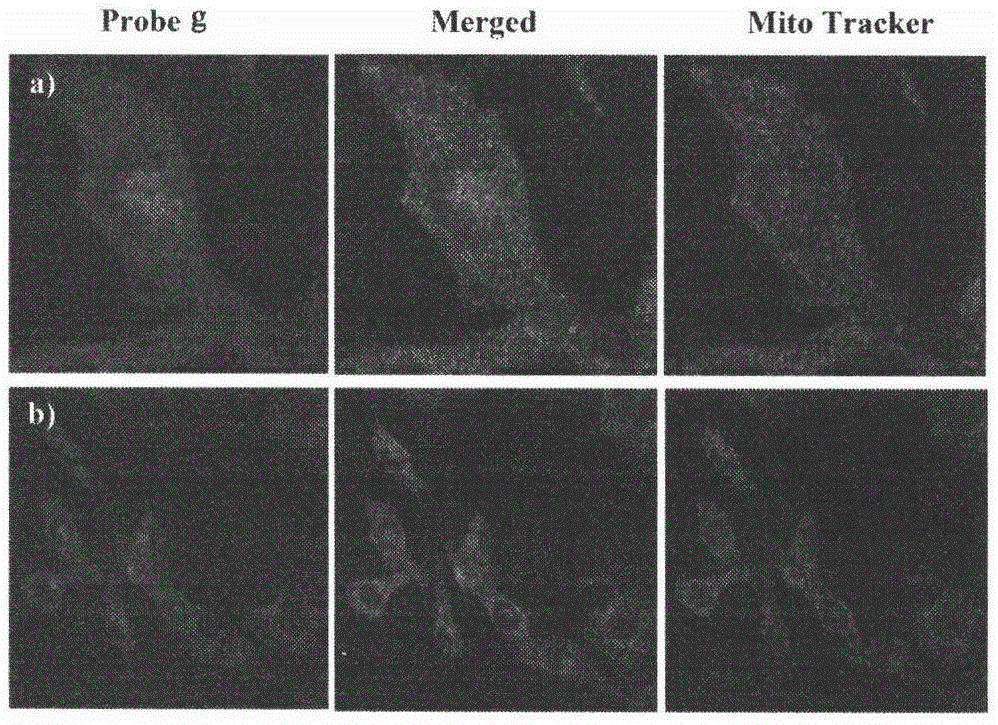
[0052] 23-HBA fluorescent probe staining of B16F10 cells
[0053] B16F10 cells in the logarithmic growth phase were selected and inoculated on glass coverslips placed in a 6-well plate, 5×10 cells per well 4 After 24 hours of attachment, compound g (2 μM, 4 μM, 10 μM and 20 μM InDMSO) was added to the complete medium and incubated at different time points (15min, 30min, 1h, 2h, 4h, 24h). After incubation for the corresponding time, the cells were fixed with 4% paraformaldehyde (in 1×PBS) for 10 minutes, and after washing with PBS, the glass coverslips were taken out for fluorescence imaging. Under the fluorescent microscope, observe the coloring part of the cells, the distribution of fluorescence and the change of brightness, etc., the results are shown in figure 2 a.
[0054] B16F10 cells in the logarithmic growth phase were selected and inoculated on glass coverslips placed in a 6-well plate, 5×10 cells per well 5 After 24 hours of attachment, 10 μM compound g was added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com