Slow-release medicinal preparation for treating heart diseases
A sustained-release drug and sustained-release preparation technology, which is applied in the field of sustained-release pharmaceutical preparations and metoprolol sustained-release drugs, can solve the problems of poor sustained-release effect, unsatisfactory demand, and low drug load, and achieve good results. The effect of application prospect, simple preparation method and controllable carrier pore size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] One. Preparation of metoprolol tartrate sustained-release preparation
[0037] (1) Preparation of spherical mesoporous silica nanoparticles (MCM-41)
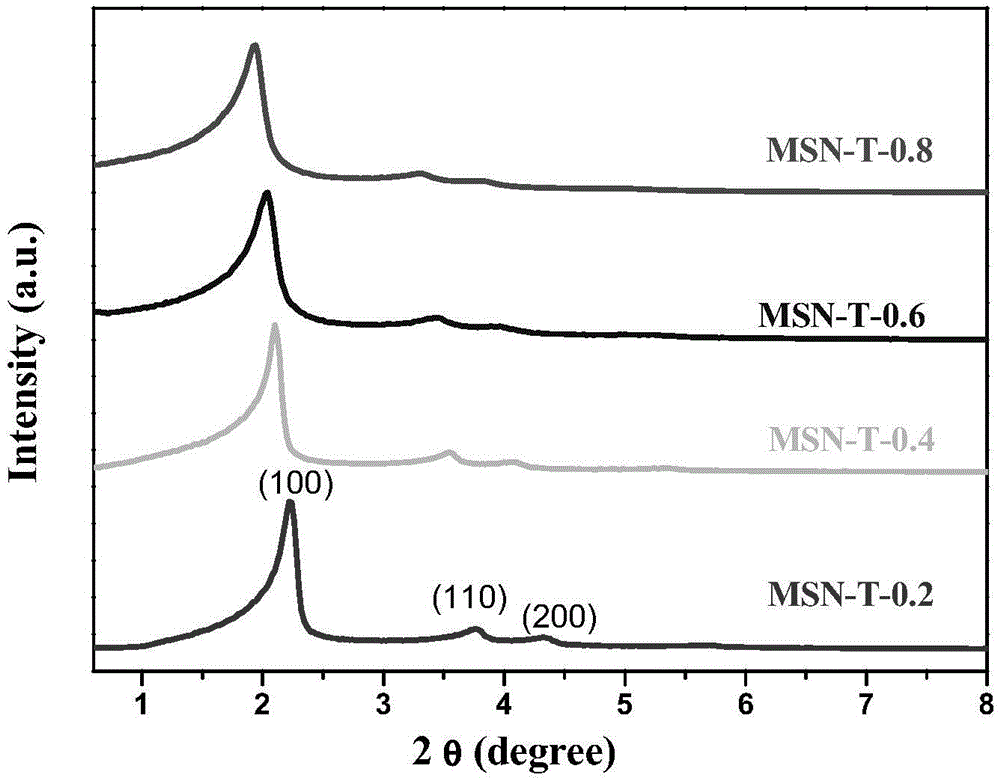
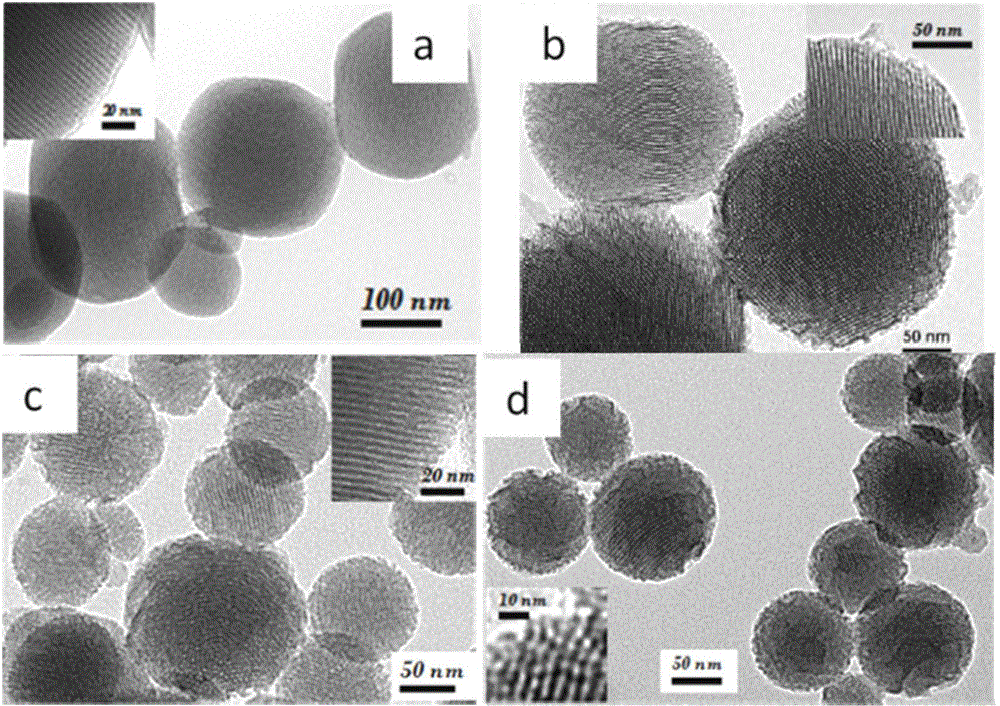
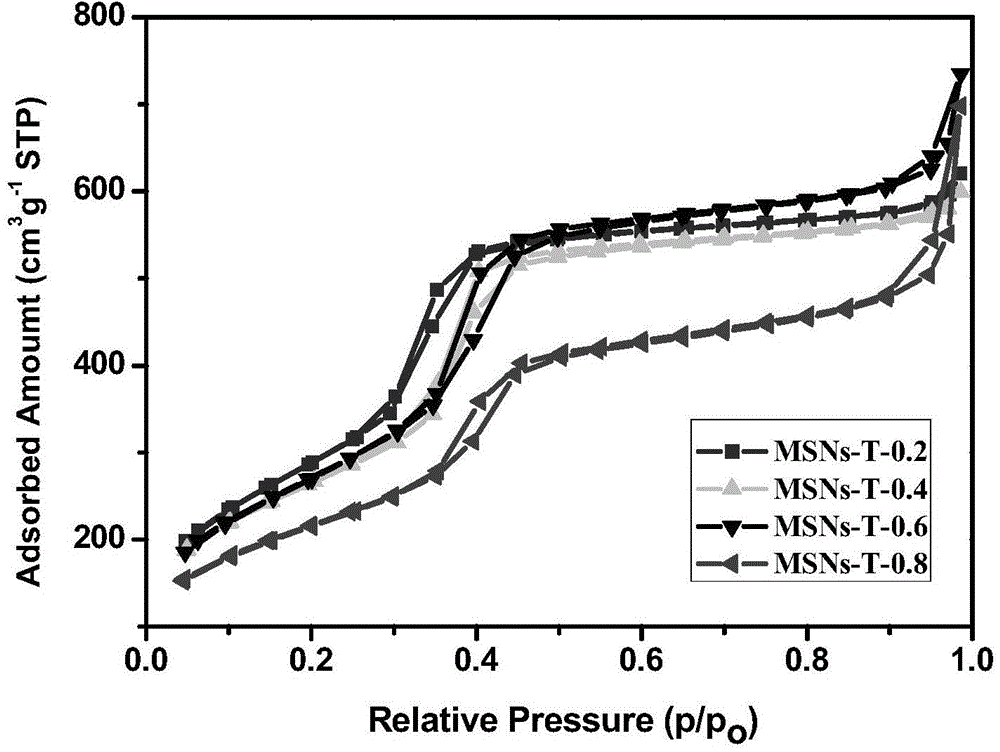
[0038] Place the three-neck flask in a water bath at 80°C, measure 240 ml of deionized water and 1.75 ml of NaOH solution with a concentration of 2 mol / L, and then add 0.5 g of cetyl ammonium bromide. After stirring, after the solution was clarified, 0.3 ml of mesitylene was added according to the molar ratio of tetraethyl orthosilicate (TEOS) to mesitylene (TMB) being 0.2. After stirring for 5 min, 2.5 ml of ethyl orthosilicate was added dropwise. After stirring for 2 hours, it was naturally cooled to obtain a white suspension, which was filtered with suction, washed with deionized water three times, and dried in a vacuum oven at 60°C. Put the obtained white powder into 200 ml of ammonium nitrate ethanol solution with a concentration of 1 g / ml and reflux at 80°C for 8 hours, filter with suction, wash with ethanol for 3...
Embodiment 2
[0044] One. Preparation of metoprolol tartrate sustained-release preparation
[0045] (1) Preparation of spherical mesoporous silica nanoparticles (MCM-41)
[0046] The three-neck flask was placed in a water bath at 80°C, 240 ml of deionized water and 1.75 ml of NaOH solution with a concentration of 2 mol / L were measured, and then 0.5 g of cetyl ammonium bromide was added. After stirring, after the solution is clarified, 0.6 ml of mesitylene is added according to the molar ratio of tetraethyl orthosilicate (TEOS) to mesitylene (TMB) being 0.4. After stirring for 5 min, 2.5 ml of ethyl orthosilicate was added dropwise. After stirring for 2 hours, it was naturally cooled to obtain a white suspension, which was filtered by suction, washed three times with deionized water and twice with ethanol, and the obtained sample was dried in a vacuum oven at 60°C. Put the obtained white powder into 200 ml of ammonium nitrate ethanol solution with a concentration of 1 g / ml and reflux at 80...
Embodiment 3
[0052] One. Preparation of metoprolol tartrate sustained-release preparation
[0053] (1) Preparation of spherical mesoporous silica nanoparticles (MCM-41)
[0054] The three-neck flask was placed in a water bath at 80°C, 240 ml of deionized water and 1.75 ml of NaOH solution with a concentration of 2 mol / L were measured, and then 0.5 g of cetyl ammonium bromide was added. After stirring, after the solution was clarified, 0.9 ml of mesitylene was added according to the molar ratio of tetraethyl orthosilicate (TEOS) to mesitylene (TMB) being 0.6. After stirring for 5 min, 2.5 ml of ethyl orthosilicate was added dropwise. After stirring for 2 hours, it was naturally cooled to obtain a white suspension, which was filtered by suction, washed three times with deionized water and twice with ethanol, and the obtained sample was dried in a vacuum oven at 60°C. Put the obtained white powder into 200 ml of ammonium nitrate ethanol solution with a concentration of 1 g / ml and reflux at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com