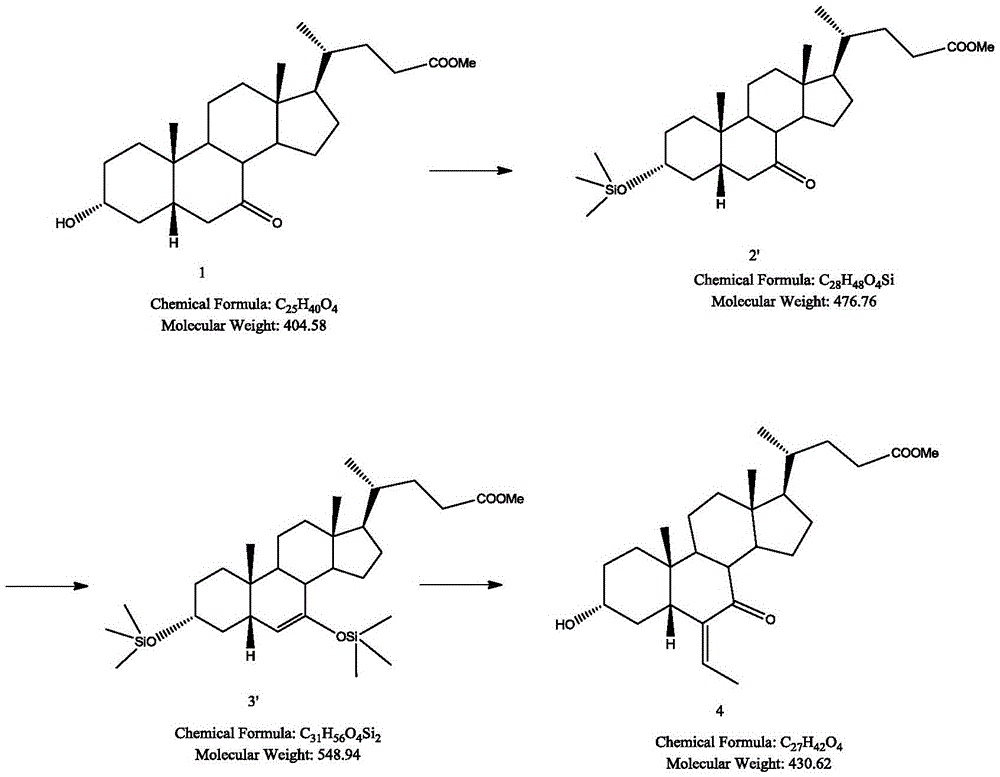
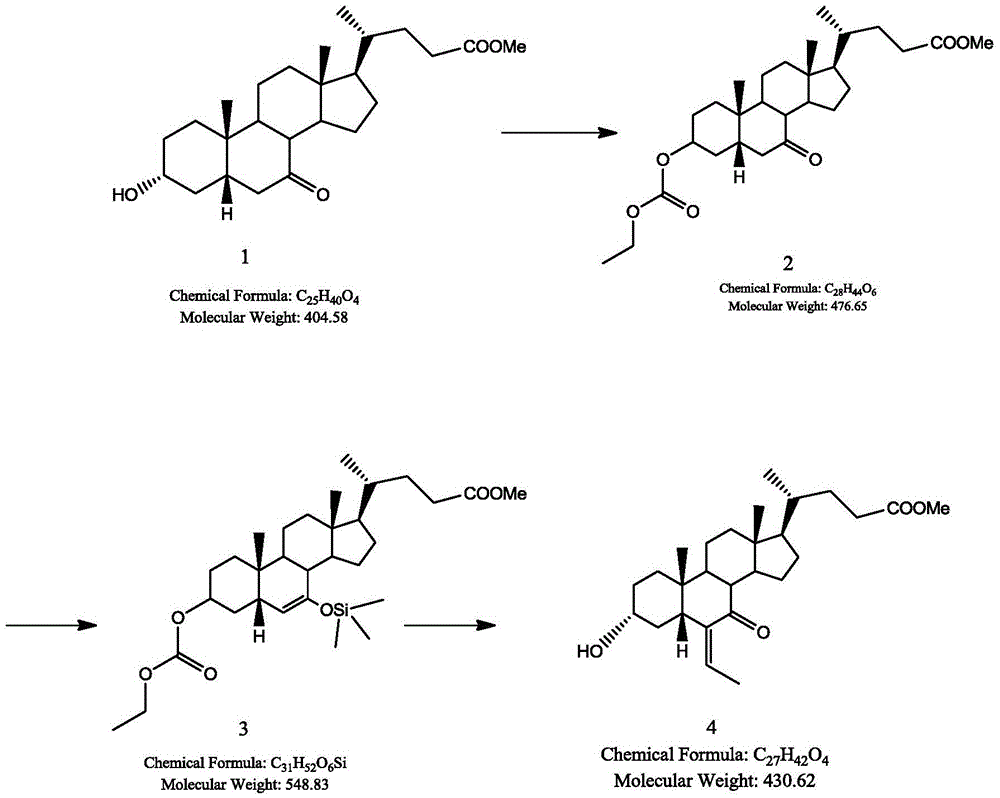
Synthetic method for obeticholic acid intermediate
A technology of obeticholic acid and synthesis method, which is applied in the field of medicine, can solve the problems of low reaction efficiency and large amount of LDA, and achieve the effect of high efficiency and reduced amount of LDA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Ethyl acetate (40 g) was charged into a reactor protected by inert gas, 3α-hydroxy-7-keto-5β-cholan-24-oic acid methyl ester (8.47 g) was dissolved therein, and 4.5 g of pyridine was added. The solution was cooled to 0°C, and ethyl chloroformate (4.0 g) was slowly added dropwise. After the ethyl chloroformate was added dropwise, the temperature of the reaction solution was raised to 22°C, and kept stirring at 22°C for 2.5h. Concentrate under reduced pressure to remove ethyl acetate. Add 34 g of ice water to dissolve the concentrate, then add 17 g, 17 g, and 8.5 g of ethyl acetate to extract the aqueous phase three times, and combine the three organic layers. The organic layer was washed with 8.5g of 10% HCl aqueous solution, 8.5g of saturated sodium bicarbonate solution, and 8.5g of saturated sodium chloride solution, and then dried with an appropriate amount of anhydrous sodium sulfate, and the organic layer was concentrated to dryness to obtain 10.1g of crude product...
Embodiment 2
[0026] Tetrahydrofuran (40 g) was charged into a reactor protected by inert gas, 3α-hydroxy-7-keto-5β-cholane-24-oic acid methyl ester (8.47 g) was dissolved therein, and 4.5 g of pyridine was added. The solution was cooled to 5°C, and propyl chloroformate (4.0 g) was slowly added dropwise. After the propyl chloroformate was added dropwise, the temperature of the reaction solution was raised to 25°C and kept at 25°C with stirring for 2h. Concentrate under reduced pressure to remove tetrahydrofuran. Add 34g of ice water to dissolve. 17g, 17g and 8.5g of ethyl acetate were added to extract the aqueous phase three times, and the three organic layers were combined. The organic layer was washed with 8.5 g of 10% HCl aqueous solution, 8.5 g of saturated sodium bicarbonate solution, and 8.5 g of saturated sodium chloride solution, and dried with an appropriate amount of anhydrous sodium sulfate. The organic layer was concentrated to dryness to obtain 10.3 g of the crude product, w...
Embodiment 3
[0028] Butyl acetate (40g) was packed into a reactor protected by an inert gas, 3α-hydroxyl-7-ketone-5β-cholan-24-acid methyl ester (8.47g) was dissolved therein, and 4.5g triethylamine was added . The solution was cooled to 2°C, and butyl chloroformate (4.0 g) was slowly added dropwise. After the butyl chloroformate was added dropwise, the temperature of the reaction solution was raised to 20°C, and kept stirring at 20°C for 3h. Concentrate under reduced pressure to remove butyl acetate. Add 34g of ice water to dissolve. 17g, 17g and 8.5g of ethyl acetate were added to extract the aqueous phase three times, and the three organic layers were combined. The organic layer was washed with 8.5 g of 10% HCl aqueous solution, 8.5 g of saturated sodium bicarbonate solution, and 8.5 g of saturated sodium chloride solution, and dried with an appropriate amount of anhydrous sodium sulfate. The organic layer was concentrated to dryness to obtain 10.2 g of the crude product, which was ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap