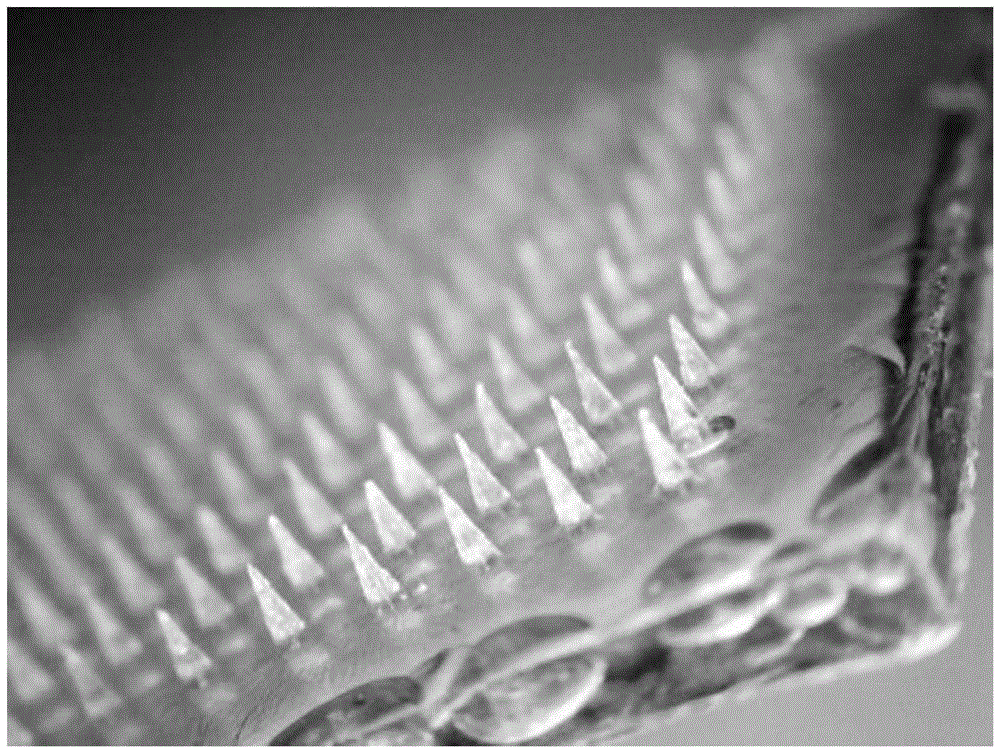
Active separation-type soluble microneedle and preparation method thereof
A soluble and microneedle technology, which is applied in the direction of medical preparations with non-active ingredients, pharmaceutical formulas, non-effective ingredients of polymer compounds, etc., can solve the problem that the time of administration depends on the dissolution rate of the needle tip, intradermal fracture waste, and ineffective administration. Adequate and other issues, to achieve the effect of convenient and effective drug release, expanding the dissolution time difference, and avoiding allergic damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This embodiment provides a soluble microneedle whose middle layer is carbomer 971 and its preparation method, the preparation method comprising the following steps:
[0036] 1) Preparation of needle tip solution
[0037] Weigh a certain amount of dextran (molecular weight 40,000), dissolve it in deionized water at a mass ratio of 1:2, and stir in a water bath at 45°C to obtain the excipient material dextran solution, namely the needle tip solution.
[0038] 2) Preparation of intermediate layer solution
[0039] A certain amount of Carbomer 971 was weighed, added into deionized water, stirred and dissolved to obtain 0.03 g / ml Carbomer gel-like substance.
[0040] 3) Preparation of base solution
[0041] Weigh a certain mass of polyvinylpyrrolidone K90, add absolute ethanol, stir to dissolve, and swell overnight to obtain a base layer solution with a concentration of polyvinylpyrrolidone K90 of 0.4 g / mL.
[0042] 4) Preparation of soluble microneedles
[0043] Inject ...
Embodiment 2
[0045] This embodiment provides a soluble microneedle whose middle layer is poloxamer 184 and its preparation method, the preparation method comprising the following steps:
[0046] 1) Preparation of needle tip solution
[0047] Weigh a certain amount of dextran (molecular weight 40,000), dissolve it in deionized water at a mass ratio of 1:2, and stir in a water bath at 45°C to obtain the excipient material dextran solution, namely the needle tip solution.
[0048] 2) Preparation of intermediate layer solution
[0049] Weigh a certain amount of chitosan hydrochloride, add it to deionized water, stir and dissolve to obtain a 0.2g / ml chitosan hydrochloride solution, weigh a certain amount of poloxamer and dissolve it in the chitosan hydrochloride solution In, 0.4 g / ml poloxamer colloidal substance was obtained.
[0050] 3) Preparation of base solution
[0051] Weigh a certain mass of polyvinylpyrrolidone K90, add absolute ethanol, stir to dissolve, and swell overnight to obta...
Embodiment 3
[0055] This embodiment provides a soluble microneedle whose middle layer is hydroxypropyl methylcellulose and a preparation method thereof, the preparation method comprising the following steps:
[0056] 1) Preparation of needle tip solution
[0057] Weigh a certain amount of dextran (molecular weight 40,000), dissolve it in deionized water at a mass ratio of 1:2, and stir in a water bath at 45°C to obtain the excipient material dextran solution, namely the needle tip solution.
[0058] 2) Preparation of intermediate layer solution
[0059] Weigh a certain amount of hydroxypropylmethylcellulose, add it into deionized water, stir and dissolve in a water bath at 30°C to obtain a 0.5 g / ml hydroxypropylmethylcellulose solution.
[0060] 3) Preparation of base solution
[0061] Weigh a certain mass of polyvinylpyrrolidone K90, add absolute ethanol, stir to dissolve, and swell overnight to obtain a base layer solution with a concentration of polyvinylpyrrolidone K90 of 0.37 g / mL. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com