O-hydroxyaniline derivative and preparation method thereof
A technology for o-hydroxyaniline and derivatives, which is applied in the field of o-hydroxyaniline derivatives and their preparation, can solve the problems of complex synthesis methods and low efficiency of aniline compounds, and achieves high synthesis efficiency, broad application prospects and simple preparation methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
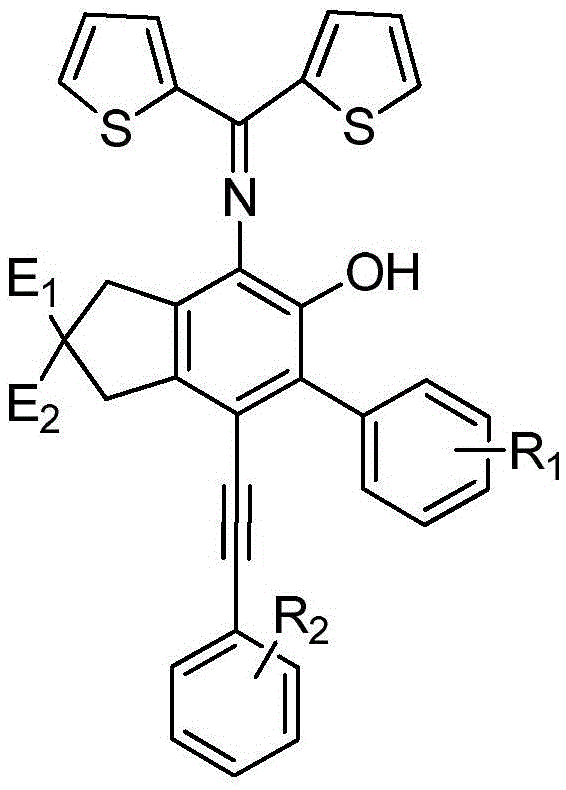
[0032] A kind of o-hydroxyaniline derivative, its structural formula is:
[0033]
[0034] A preparation method of o-hydroxyaniline derivatives, comprising the following steps:
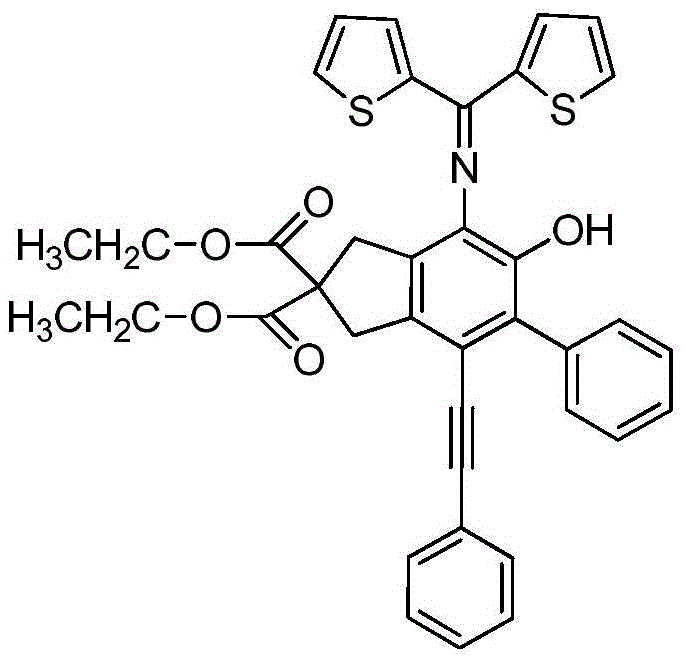
[0035] (1) With 820mmol sodium hydride as a catalyst, 200mmol diethyl malonate and 440mmol propargyl bromide were added to 250mL anhydrous acetonitrile in an ice-water bath, stirred and reacted for 8 hours, the product was washed with water, extracted with ethyl acetate, and Press and spin dry, and use ethyl acetate:petroleum ether column chromatography with a volume ratio of 1:100 to obtain a white solid product, namely compound 1;
[0036] (2) Mix 80mmol of compound 1 with 200mmol of phenylethynyl bromide in 1.09mmol of Pd (PPh 3 ) 2 Cl 2 / CuI anhydrous and oxygen-free catalytic system, the molar ratio Pd(PPh 3 ) 2 Cl 2 : CuI=3:1, with 340mmol triethylamine as base, with 200ml anhydrous acetonitrile as solvent, stirring and reacting at room temperature for 12 hours, the product was washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com