Magnetic cellulose-dimethyl imidazole zinc salt and preparing method and application thereof
A technology of dimethylimidazole zinc and dimethylimidazole, which is applied in the field of magnetic cellulose-dimethylimidazole zinc salt and its preparation, can solve the problems of difficult recycling and reuse, loss of enzyme activity, etc., and achieve the benefit of recycling and recycling. Easy to use and operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
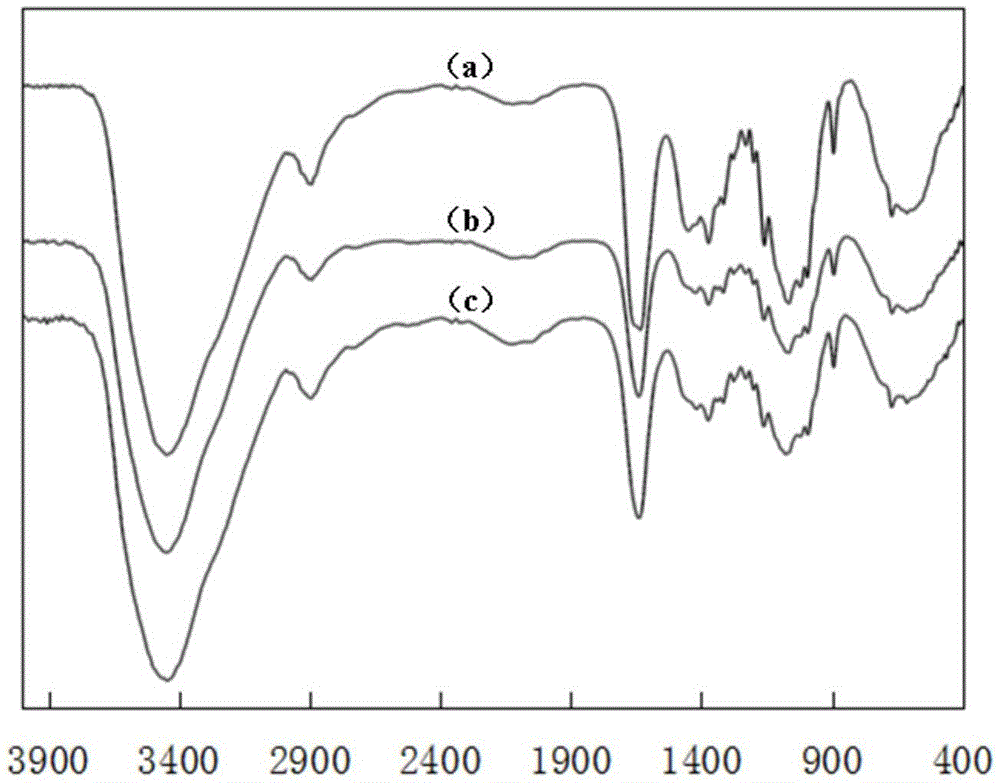
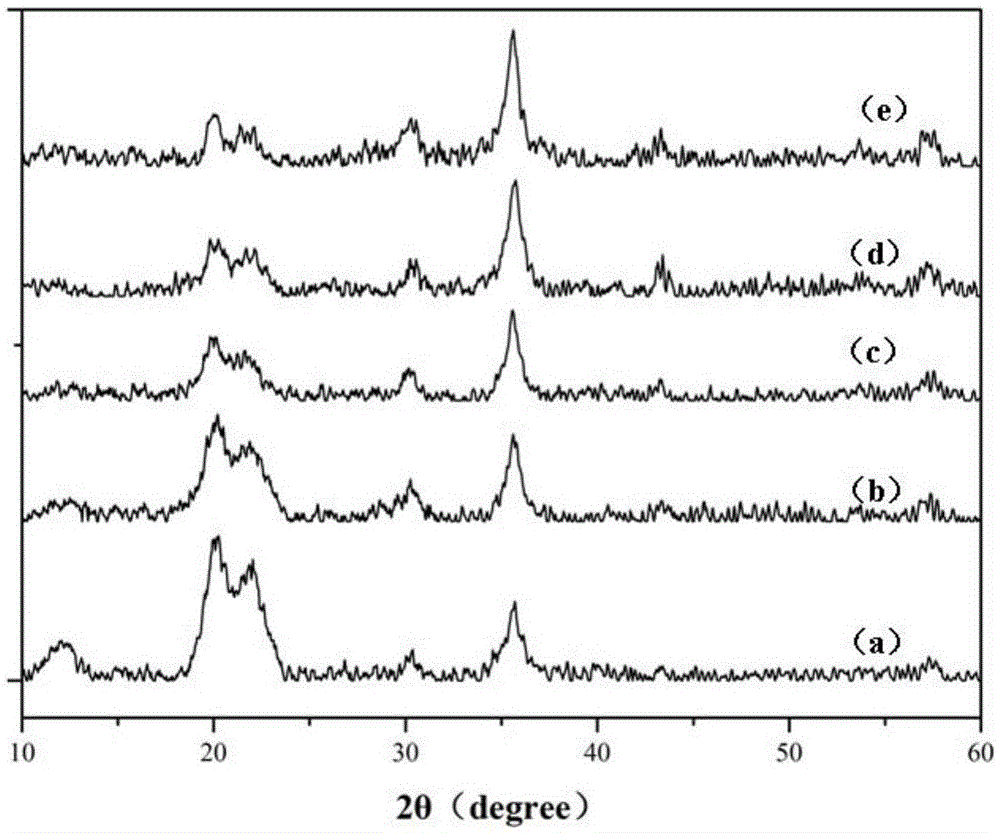
Image
Examples
Embodiment 1
[0038] (1) Weigh 2.43gFeCl 3 ·6H 2 O and 0.9gFeCl 2 4H 2 O was dissolved in 300mL distilled water, ammonia water was added dropwise to the solution, and the pH of the solution was adjusted to 10; the mixed solution was reacted at 30°C for 1h to obtain nano-Fe 3 o 4 ; Prepared nano-Fe 3 o 4 It can be stored in Tris-HCl buffer solution with pH=7;
[0039] (2) Weigh 10 mg of microcrystalline cellulose and dissolve it in an aqueous NaOH-Urea solution containing 7% NaOH and 12% Urea by mass at -12°C, wherein the mass ratio of NaOH-Urea solution to cellulose is 1 : 0.02; Add 5 mg of Fe under magnetic stirring conditions 3 o 4 , then add water to the mixed solution to regenerate the cellulose, separate it with a magnet, and wash it three times with distilled water to obtain magnetic cellulose nanocrystals;
[0040] (3) 23.799 mg of zinc nitrate hexahydrate was dissolved in 2 mL of distilled water, mixed with 15 mg of the magnetic cellulose nanocrystals prepared in step (1) a...
Embodiment 2
[0045] (1) Weigh 2.43gFeCl 3 ·6H 2 O and 0.9gFeCl 2 4H 2 O was dissolved in 300mL distilled water, ammonia water was added dropwise to the solution, the pH of the solution was adjusted to 10, and the mixed solution was reacted at 30°C for 1h to obtain nano-Fe 3 o 4 ; Prepared nano-Fe 3 o 4 It can be stored in Tris-HCl buffer solution with pH=7;
[0046] (2) Weigh 10 mg of microcrystalline cellulose and dissolve it in an aqueous NaOH-Urea solution containing 7% NaOH and 12% Urea by mass at -12°C, wherein the mass ratio of NaOH-Urea solution to cellulose is 1 : 0.02, add 10mg of Fe under the condition of magnetic stirring 3 o 4 , then add water to the mixed solution to regenerate the cellulose, separate it with a magnet, and wash it three times with distilled water to obtain magnetic cellulose nanocrystals;
[0047] (3) 23.799 mg of zinc nitrate hexahydrate was dissolved in 2 mL of distilled water, mixed with 20 mg of magnetic cellulose nanocrystals prepared in step (1) a...
Embodiment 3
[0052] (1) Weigh 2.43gFeCl 3 ·6H 2 O and 0.9gFeCl 2 4H 2 O was dissolved in 300mL distilled water, ammonia water was added dropwise to the solution, the pH of the solution was adjusted to 10, and the mixed solution was reacted at 30°C for 1h to obtain nano-Fe 3 o 4 ; Prepared nano-Fe 3 o 4 It can be stored in Tris-HCl buffer solution with pH=7;
[0053] (2) Weigh 10 mg of microcrystalline cellulose and dissolve it in an aqueous NaOH-Urea solution containing 7% NaOH and 12% Urea by mass at -12°C, wherein the mass ratio of NaOH-Urea solution to cellulose is 1 : 0.02, add 15mg of Fe under the condition of magnetic stirring 3 o 4 , then add water to the mixed solution to regenerate the cellulose, separate it with a magnet, and wash it three times with distilled water to obtain magnetic cellulose nanocrystals;
[0054] (3) 23.799 mg of zinc nitrate hexahydrate was dissolved in 2 mL of distilled water, and 25 mg of the magnetic cellulose nanocrystals prepared in step (1) we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com