Fluorescent protein iRFP-based dimolecular fluorescent fragment complementary system, and applications
A fluorescent protein and bimolecular technology, applied in the field of bimolecular fluorescent fragment complementary systems, can solve the problems of inability to intuitively display the inhibition effect, lack of screening and verification methods, etc., and achieve the effect of simple inhibition effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A construction of a bimolecular fluorescent fragment complementation system based on the fluorescent protein iRFP97-98 site, the construction process includes the following steps:
[0045] (1) PCR amplification (upstream Primer: 5'-GAAGATCTATGGCTGAAGGATCCGTC-3', downstream primer: 5'-AAGGTACCCATCGTGAAGCCGACAGTG-3') to obtain the gene sequence of the nitrogen-terminal fragment iRN97 of the fluorescent protein iRFP from bacteria, and use the double restriction sites BglII and KpnI to convert the iRN97 gene Inserted into the multiple cloning site of the pEGFP-C1 (purchased from Clontech) vector to construct the vector pEGFP-iRN97;
[0046] (2), from the plasmid pcDNA3.1-iRFP by PCR amplification (upstream primer: 5'-GAAGATCTATGATGCGAAAGGACG-3', downstream primer: 5'-CCCAAGCTTCCTCTTCCATCACGCCGATCTGCCAGG-3') to obtain the carbon-terminal fragment of the fluorescent protein iRFP derived from bacteria For the gene sequence of iRC98, the iRC98 gene was inserted into the multip...
Embodiment 2
[0053] A bimolecular fluorescent fragment complementation system based on fluorescent protein iRFP, the construction process includes the following steps:
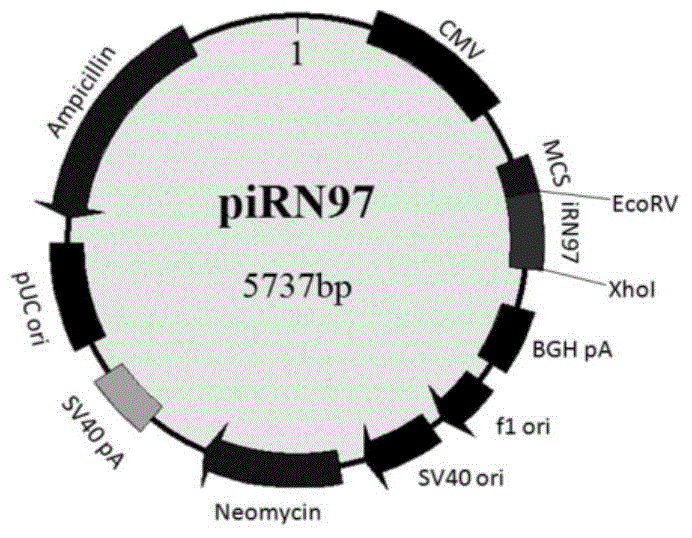
[0054] 1) Obtain the gene sequence of the nitrogen-terminal fragment iRN97 of the bacterial fluorescent protein iRFP from the plasmid pEGFP-iRN97 by PCR amplification (upstream primer: 5'-GGGATATCGAGCCACCCCCTCCTATGGCTGAAGGATCCGTC-3', downstream primer: 5'-CCGCTCGAGTTACATCGTGAAGCCGACAG-3') (shown in SEQIDNO.2), utilize the double restriction site EcoRV and XhoI, iRN97 gene is inserted on the multiple cloning site of pcDNA3.1 vector, namely plasmid piRN97( Figure 1-A );
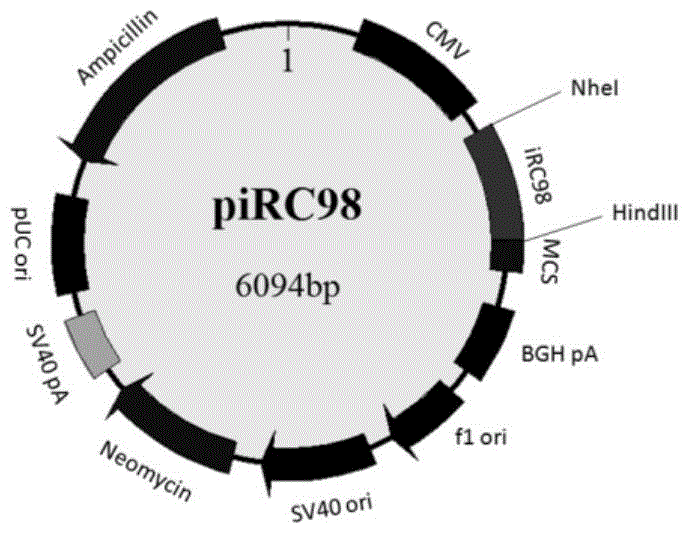
[0055] 2) Obtain the gene sequence of the carbon-terminal fragment iRC98 of the bacterial fluorescent protein iRFP from the plasmid pEGFP-iRC98 by PCR amplification (upstream primer: 5'-CGGCTAGCGCCACCATGCGAAAGGACGCAGGCTTCATC-3', downstream primer: 5'-CCCAAGCTTCCTCTTCCATCACGCCGATCTGCC-3') (shown in SEQIDNO.3), utilize double-digestion site NheI and HindIII,...
Embodiment 3
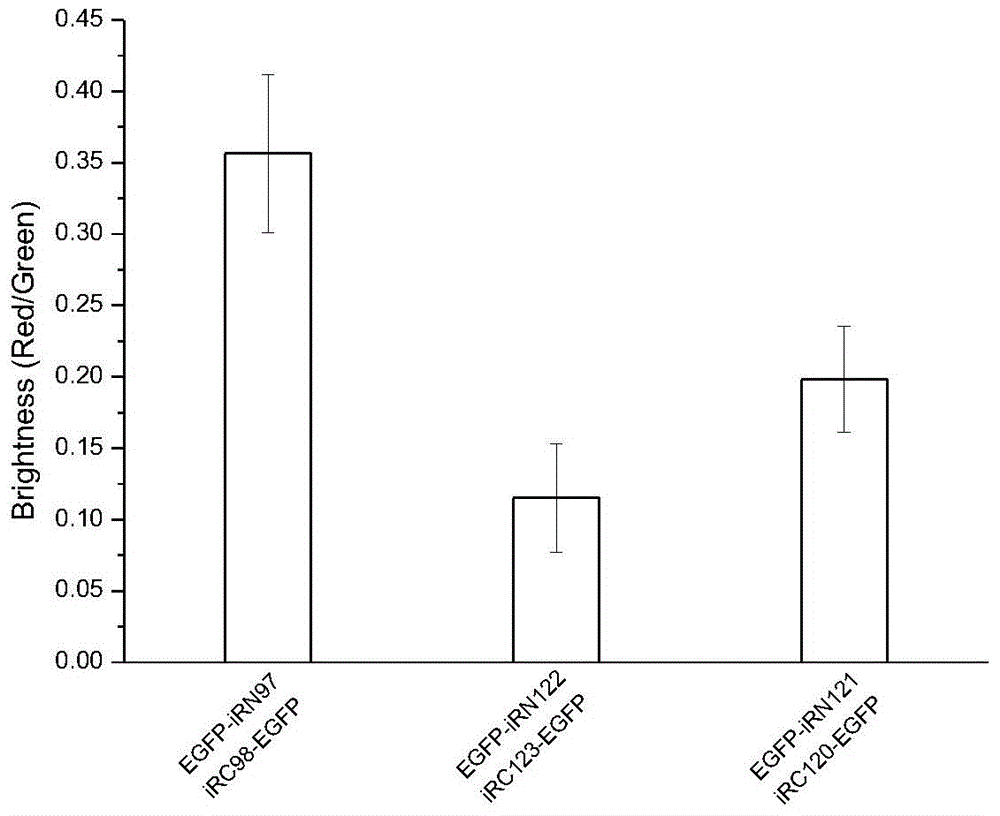
[0057] The application of a fluorescent protein iRFP-based bimolecular fluorescent fragment complementation system in the study of protein interactions, the application process is as follows:
[0058] 1) The synthetic bJun gene sequence (synthesized by Shanghai Sangong, refer to the literature HuCDetal.MolCell.2002; 9:789-98.), using the double restriction sites NheI and HindIII, inserting the bJun gene into the multiple clone of the piRN97 vector site, construct the vector pbJun-iRN97;
[0059] 2) The gene sequence of the synthetic bFos (synthesized by Shanghai Sangong, refer to the literature HuCDetal.MolCell.2002; 9:789-98.), using the double restriction site KpnI and NotI, insert the bFos gene into the multiple clone of the plasmid piRC98 site, construct the vector piRC98-bFos;
[0060] 3) The control group is a plasmid constructed by using the upstream primer 5'-GGGGTACCGAGCCACCCCCTCCTATGGGTCGTGCGCAGTC-3' and the downstream primer 5'-ATTTGCGGCCGCTTAACCCAGGTCGTTCGGGATTTTG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com