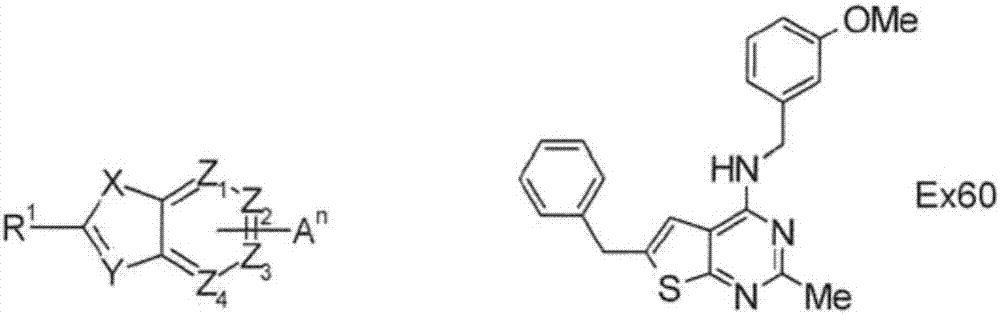
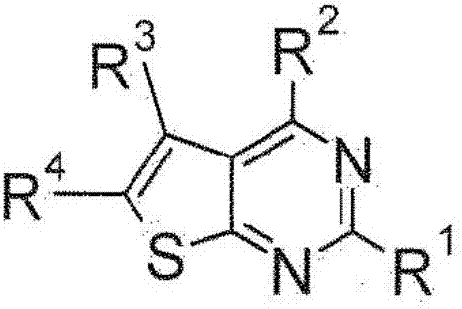
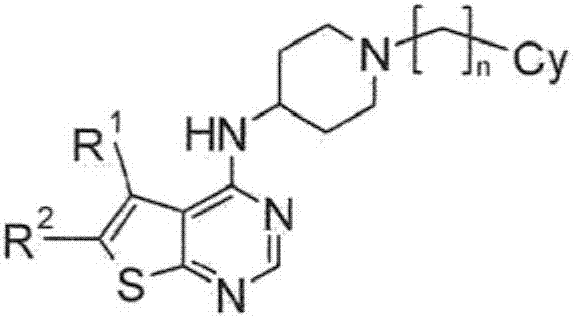
Sulfur-containing bicyclic compounds
A compound, the technology of dimethylcyclohexane, which is applied in the field of sulfur-containing bicyclic compounds, can solve the problems of narrowing of the therapeutic window and limitation of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0325] Hereinafter, the production method of the compound of formula (I) is demonstrated in further detail based on an Example. It should be noted that the present invention is not limited to the compounds described in the following examples. In addition, the production method of the raw material compound is shown in the production example. In addition, the production method of the compound of formula (I) is not limited to the production method of the specific examples shown below, and the compound of formula (I) can also be obtained by a combination of these production methods, or those skilled in the art method manufacturing.
[0326] The compounds shown in the Tables described below were produced by the above-mentioned production methods and methods obvious to those skilled in the art, or modified methods of these methods. In the table, the structures and physicochemical data of these example compounds and their production methods are shown. It should be noted that the s...
manufacture example 3
[0333] 2M NaOH aqueous solution (200mL) was added to a mixture of 2-acetamido-5-(4,4-dimethylcyclohexyl)thiophene-3-carboxamide (37.3g) and EtOH (200mL), at 80°C Heat and stir for 2 hours. After allowing the reaction mixture to cool to room temperature, 1M hydrochloric acid (500 mL) was added, followed by stirring at room temperature. The precipitate was collected by filtration to obtain 6-(4,4-dimethylcyclohexyl)-2-methylthieno[2,3-d]pyrimidin-4(3H)-one (26.3 g).
manufacture example 4
[0335] Add phosphorus oxychloride (14mL) and DMF ( 200 μL), heated to reflux at 150°C for 14 hours. After cooling the reaction mixture to room temperature, it was concentrated under reduced pressure. Chloroform, water and saturated sodium bicarbonate water were added to the residue and stirred. The reaction mixture was extracted with chloroform. The organic layer was washed successively with water and brine. Add MgSO to the organic layer 4 , activated carbon (2g), silica gel (100mL) and stirring, Celite filtered, concentrated under reduced pressure to obtain 4-chloro-6-cyclohexyl-2-methylthieno[2,3-d]pyrimidine (27.4g) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com