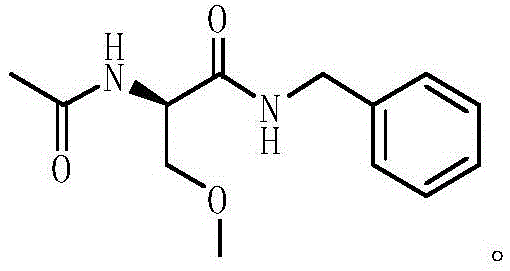
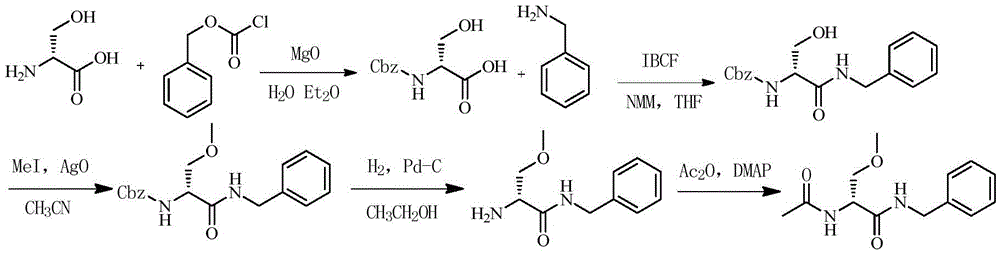
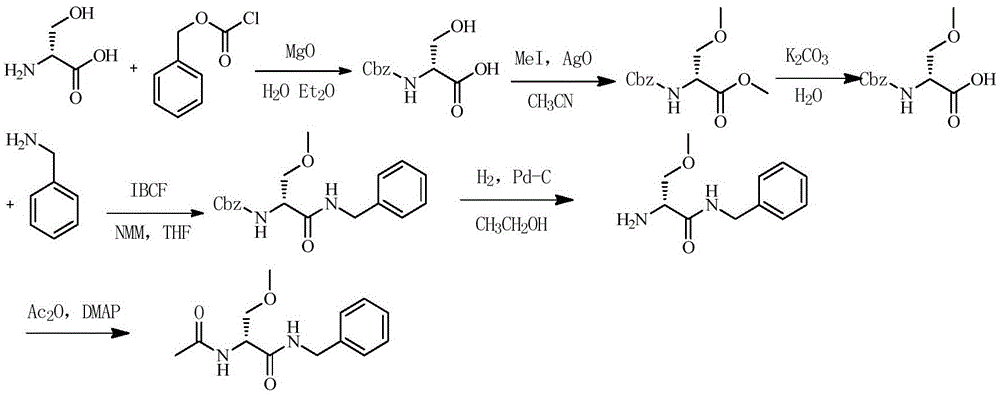
Lacosamide synthesis method
A technology of lacosamide and synthesis method, which is applied in the field of medicine, can solve problems such as high cost, expensive raw materials, and cumbersome processes, and achieve the effects of reducing emissions, making the process route green and environmentally friendly, and simplifying the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 (the synthesis of R-2-isobutoxycarbonyl-N-benzyl-3-hydroxyl propionamide)
[0045]
[0046] Add 50g of D-serine into 200ml of dichloromethane, cool down to -15°C, add 96.2g of triethylamine dropwise, stir for 10min after dropping, add 130g of isobutyl chloroformate dropwise, stir for 10min after dropping, slowly add 51g of benzyl After the amine was added and reacted for 2 hours, the dichloromethane phase was washed with 200ml of water, 200ml of 1mol / L HCl solution, 200ml of 8% sodium bicarbonate solution, and 200ml of water in sequence, dried over anhydrous sodium sulfate, and filtered with suction, and the filtrate was directly used in the next reaction .
Embodiment 2
[0047]Example 2 (synthesis of R-2-isobutoxycarbonyl-N-benzyl-3-methoxypropionamide)
[0048]
[0049] Add dropwise 200ml of ether solution containing 20.5g of diazomethane to the above dichloromethane solution under ice bath, after the dripping is completed, rise to room temperature and react for 5h. for the next reaction.
Embodiment 3
[0050] Embodiment 3 (the synthesis of R-2-amino-N-benzyl-3-methoxy propionamide)
[0051]
[0052] Add 120ml of concentrated hydrochloric acid dropwise to the above dichloromethane phase, control the temperature at 20-30°C, and react for 2 hours after dropping. 300ml of water was extracted, and the aqueous phase was adjusted to pH 12 with 30% aqueous sodium hydroxide solution, and the aqueous phase was extracted with 300ml of dichloromethane, and the dichloromethane phase was dried with anhydrous sodium sulfate and filtered with suction, and the filtrate was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com