Silver sulfide/titanium dioxide nanobelt photocatalyst and preparation method thereof
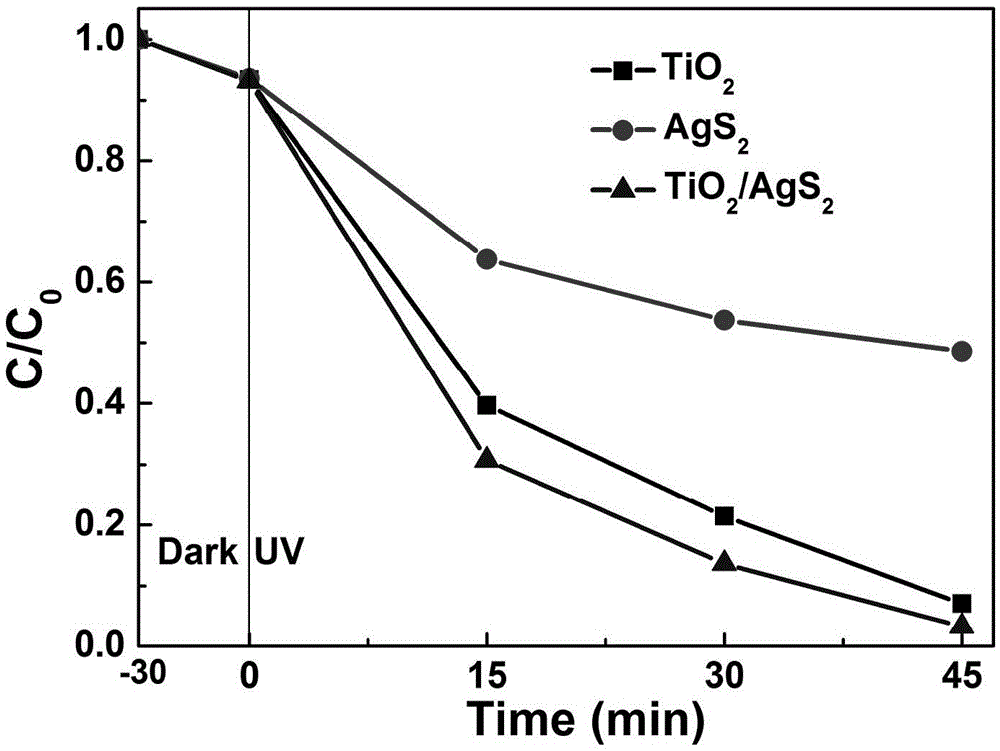
A titanium dioxide and photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem that titanium dioxide photocatalyst can only absorb ultraviolet light, etc., to achieve excellent photocatalytic effect, good light Catalytic activity and the effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
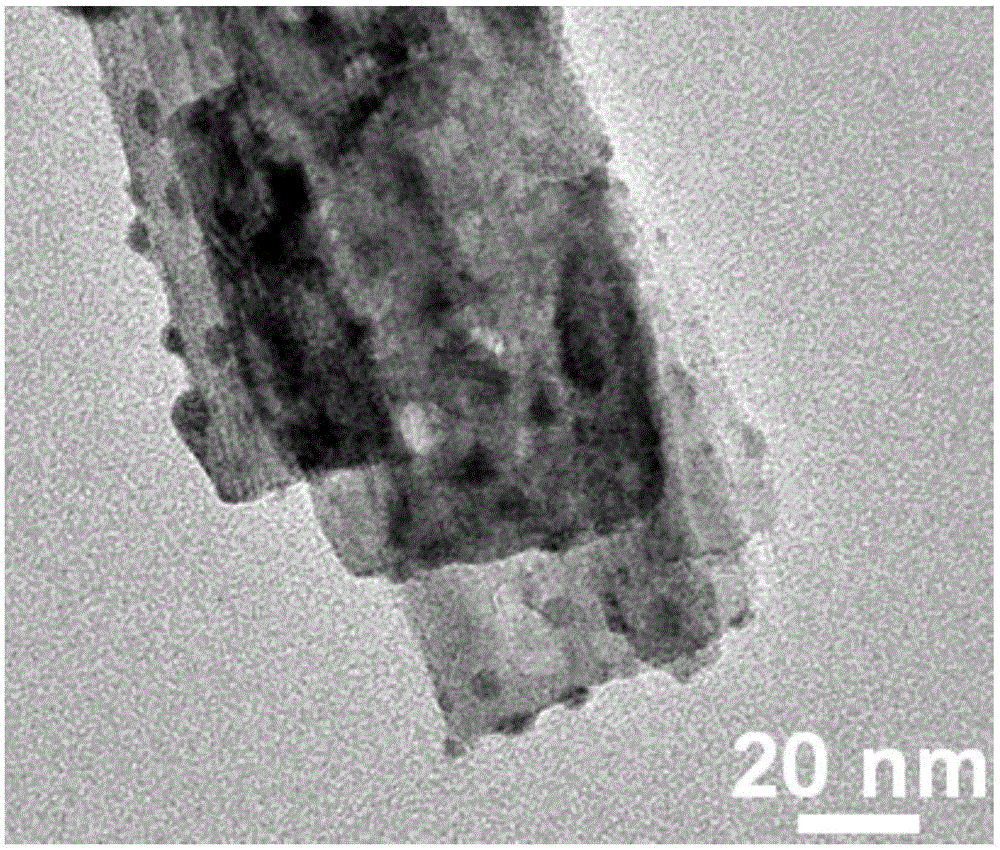
[0024] First, measure 1 mole of silver nitrate solution, weigh 5 moles of titanium dioxide nanobelts, add the silver nitrate solution and titanium dioxide nanobelts into deionized water and fully dissolve to prepare the first mixed solution. Then measure 0.5 mole of sodium sulfide solution, add the sodium sulfide solution dropwise into the first mixed solution, and then add the first mixed solution with the sodium sulfide solution dropwise for 2 hours by magnetic stirring to prepare the second mixed solution. Next, the second mixed solution was placed in a hydrothermal reaction tank, and reacted at 90° C. for 10 hours to prepare a third mixed solution. Then filter the third mixed solution to obtain a precipitate, wash the obtained precipitate with deionized water, and then vacuum-incubate the washed precipitate at 59-61°C for 9-11 hours, and finally take out the prepared Silver sulfide / titanium dioxide nanobelt photocatalyst.
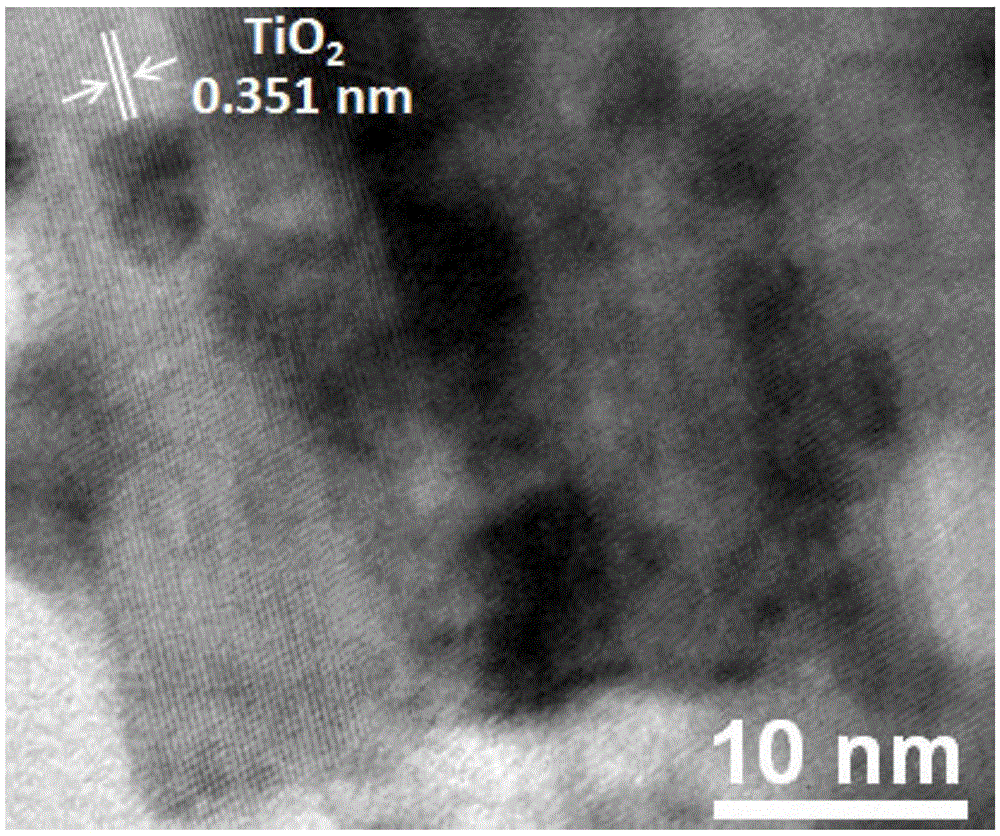
[0025] The silver sulfide / titanium dioxide nanob...
Embodiment 2
[0027] First, measure 1 mole of silver nitrate solution, weigh 2 moles of titanium dioxide nanobelts, add the silver nitrate solution and titanium dioxide nanobelts into deionized water and fully dissolve to prepare the first mixed solution. Then measure 0.5 mole of sodium sulfide solution, add the sodium sulfide solution dropwise to the first mixed solution, and then add the first mixed solution containing the sodium sulfide solution dropwise for 2 hours by magnetic stirring to prepare the second mixed solution. Then, the second mixed solution was placed in a hydrothermal reaction tank, and reacted at 90° C. for 10 hours to prepare a third mixed solution. Then filter the third mixed solution to obtain a precipitate, wash the obtained precipitate with deionized water, and then vacuum-incubate the washed precipitate at 59-61°C for 9-11 hours, and finally take out the prepared vulcanized Silver / TiO2 nanoribbon photocatalyst.
[0028] The silver sulfide / titanium dioxide nanobelt...
Embodiment 3
[0030] First, measure 1 mole of silver nitrate solution, weigh 1 mole of titanium dioxide nanobelts, add the silver nitrate solution and titanium dioxide nanobelts into deionized water and fully dissolve to prepare the first mixed solution. Then measure 0.5 mole of sodium sulfide solution, add the sodium sulfide solution dropwise to the first mixed solution, and then add the first mixed solution containing the sodium sulfide solution dropwise for 2 hours by magnetic stirring to prepare the second mixed solution. Then, the second mixed solution was placed in a hydrothermal reaction tank, and reacted at 90° C. for 10 hours to prepare a third mixed solution. Then filter the third mixed solution to obtain a precipitate, wash the obtained precipitate with deionized water, and then vacuum-incubate the washed precipitate at 59-61°C for 9-11 hours, and finally take out the prepared vulcanized Silver / TiO2 nanoribbon photocatalyst.
[0031] The silver sulfide / titanium dioxide nanobelt ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com