Method for preparing CH4 hydrate inhibitor and application thereof
A hydrate and kinetic inhibitor technology, which is applied in the fields of natural gas transportation and chemical industry, can solve the problems of short inhibition time and low dosage, and achieve the effects of convenient implementation, low cost and reduced nucleation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
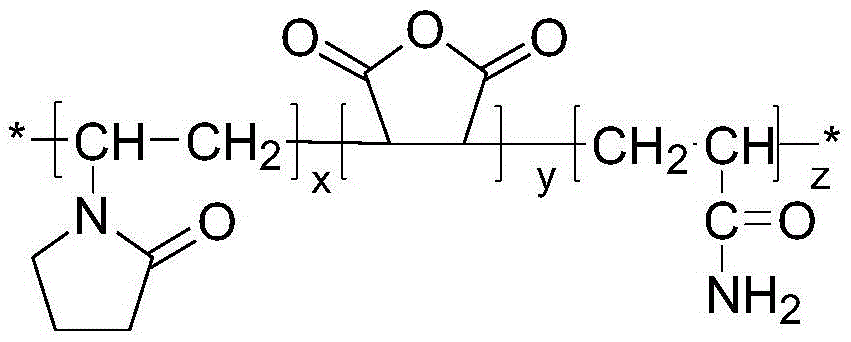
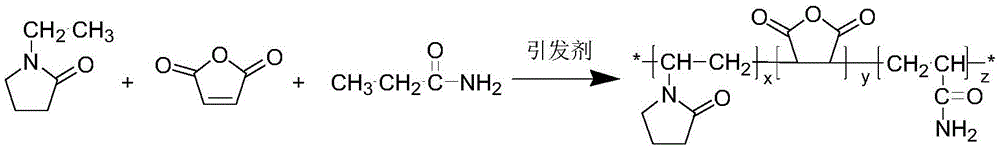
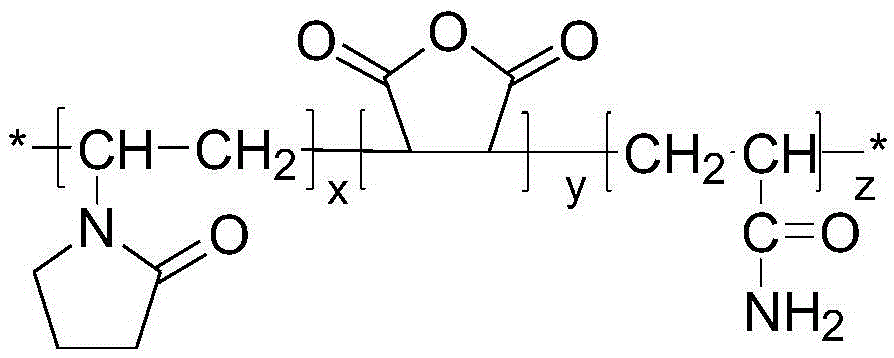
[0024] Under nitrogen protection, 8 g of N-vinylpyrrolidone, 4 g of maleic anhydride and 4 g of acrylamide were dissolved in 40 g of absolute ethanol. Add 0.4 g of azobisisobutyronitrile as an initiator at 60° C. and stir evenly for 30 minutes. Stirring was continued and the temperature was raised to 80° C. for 8 hours. Cool down to room temperature after the reaction, dry the reaction product in an oven at 45°C to constant weight, dissolve the crude product in tetrahydrofuran, wash it with n-hexane, and finally dry it in a vacuum oven at 45°C to obtain the product. Molecular weight Mn=12000, relative molecular mass distribution coefficient 2.51.
Embodiment 2
[0026] Under nitrogen protection, 8 g of N-vinylpyrrolidone, 6 g of maleic anhydride and 6 g of acrylamide were dissolved in 30 g of absolute ethanol. Add 0.8 g of azobisisobutyronitrile at 60° C. and stir evenly for 30 minutes. Stirring was continued and the temperature was raised to 90° C. for 8 hours. Cool down to room temperature after the reaction, dry the reaction product in an oven at 45°C to constant weight, dissolve the crude product in tetrahydrofuran, wash it with n-hexane, and finally dry it in a vacuum oven at 45°C to obtain the product. Molecular weight Mn=20000, relative molecular mass distribution coefficient 2.6.
Embodiment 3
[0028] Prepare 1000 g of a solution containing terpolymer P (NVP / MAH / AM) with a mass concentration of 0.11%, rinse the autoclave with distilled water, inject 170 g of the solution into the autoclave, control the temperature in the autoclave to 2.5°C, and feed CH 4 Gas, when the pressure in the kettle is 7MPa, stop the air intake, turn on the stirring, and the stirring speed is 500r / min. After the experiment, it can be seen from the curve of pressure P versus time during the experiment that at the beginning due to CH 4 The gas dissolves in water, and the pressure drops slightly. When the time is 150 minutes, the curve of pressure P versus time shows an inflection point, which proves that a large amount of hydrates begin to form. At this time CH 4 The induction time of hydrate formation under the above conditions is 150min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com