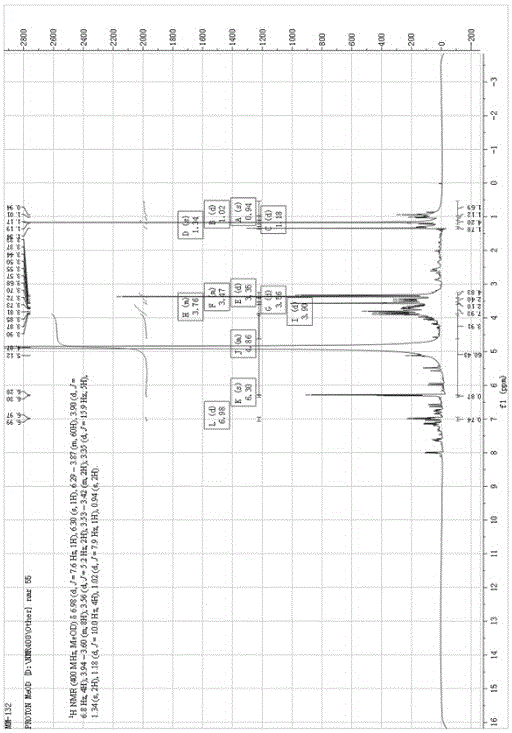
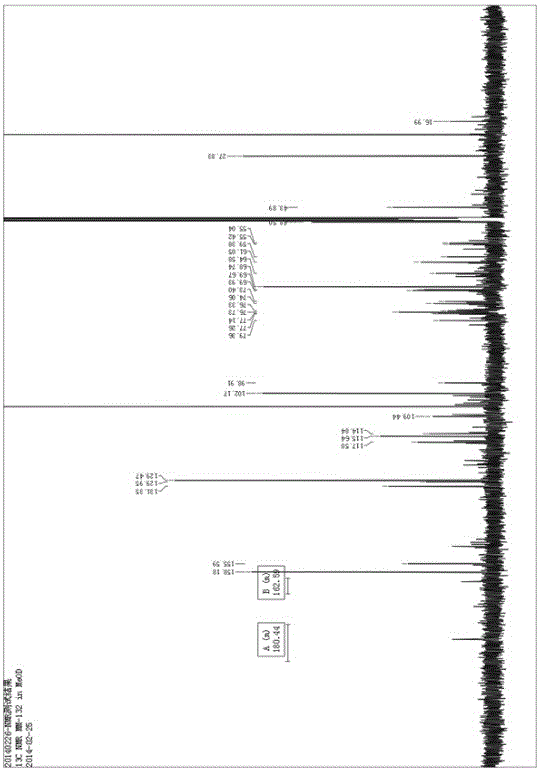
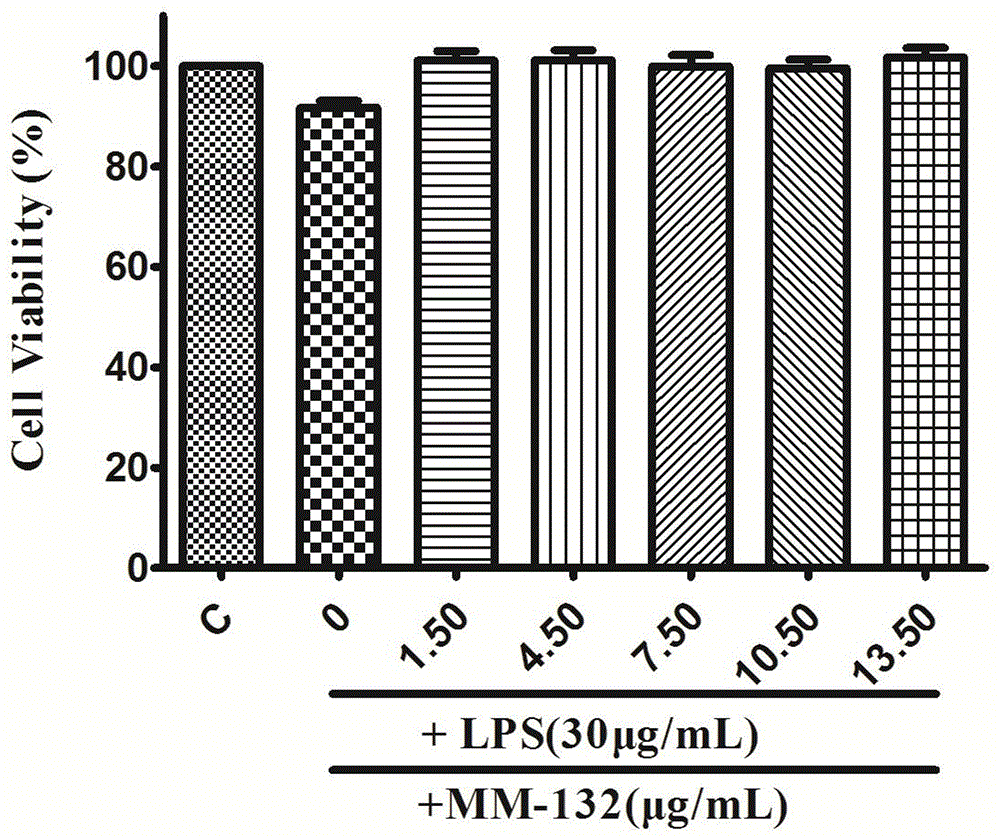
Application of phenylpropanoid compound and medically-acceptable salt thereof in preparing medicine for treating inflammatory diseases
A technology for inflammatory diseases and compounds, applied in the field of medicine, can solve problems such as complex biological effects, and achieve the effects of high purity, simple structure, and simple extraction and separation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Preparation of phenylpropanoids
[0058] This embodiment provides a method for preparing the phenylpropanoid compound represented by formula (I), which includes the following steps:
[0059] S1. Take 50 kg of big leaves, take the roots as raw materials, dry, and cut into small pieces. Reflux with 8 times the amount of 60% ethanol for 3 times, 2 hours each time, combine the extracts, concentrate until there is no alcohol taste, and obtain the extract for use;
[0060] S2. Dissolve the concentrated extract in step S1 in 10L of water, and use D101 macroporous adsorption resin column to elute it, the eluent is water, elute 3 column volumes, collect the eluent and name it MM -1, spare;
[0061] S3. The fraction MM-1 collected in step S2 is eluted by reversed-phase ODS column chromatography. The eluent is a methanol-water system with a volume ratio of 25:75, and 18 column volumes are eluted. Collect the eluent of one fraction for every 3 column volumes, and collect 6 frac...
Embodiment 2
[0064] Example 2 Preparation of phenylpropanoids
[0065] This embodiment provides a method for preparing the phenylpropanoid compound represented by formula (I), which includes the following steps:
[0066] S1. Take 40kg of big leaves, take root as raw material, dry, and cut into small pieces. Reflux with 6 times the amount of 50% ethanol for 2 times, 1 hour each time, combine the extracts, concentrate until there is no alcohol taste, and obtain the extract for later use;
[0067] S2. Dissolve the concentrated extract in step S1 in 5L of water, and use D101 macroporous adsorption resin column to elute it. The eluent is ethanol and the volume ratio of water is 15:85, and the elution is 3 column volumes. , Collect the eluate and name it MM-1 for use;
[0068] S3. The fraction MM-1 collected in step S2 is eluted by reversed-phase ODS column chromatography. The eluent is a methanol-water system with a volume ratio of 20:80, and 18 column volumes are eluted. Collect the eluent of one fr...
Embodiment 3
[0071] Example 3 Preparation of phenylpropanoids
[0072] This embodiment provides a method for preparing the phenylpropanoid compound represented by formula (I), which includes the following steps:
[0073] S1. Take 60 kg of big leaves, take root as raw material, dry, and cut into small pieces. Seven times the amount of 70% ethanol was refluxed and extracted 4 times for 3 hours each time, the extracts were combined and concentrated until there was no alcohol taste, and the extract was obtained for use;
[0074] S2. Dissolve the concentrated extract in step S1 in 8L of water, and use D101 macroporous adsorption resin column to elute it. The eluent is ethanol and the volume ratio of water is 10:90, and the elution is 3 column volumes. , Collect the eluate and name it MM-1 for use;
[0075] S3. The fraction MM-1 collected in step S2 is eluted by reversed-phase ODS column chromatography. The eluent is a methanol-water system with a volume ratio of 30:70, and 18 column volumes are eluted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com