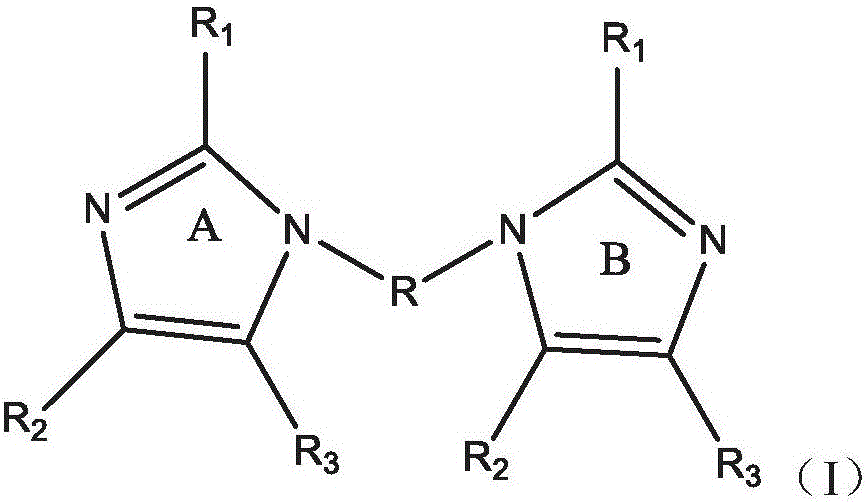
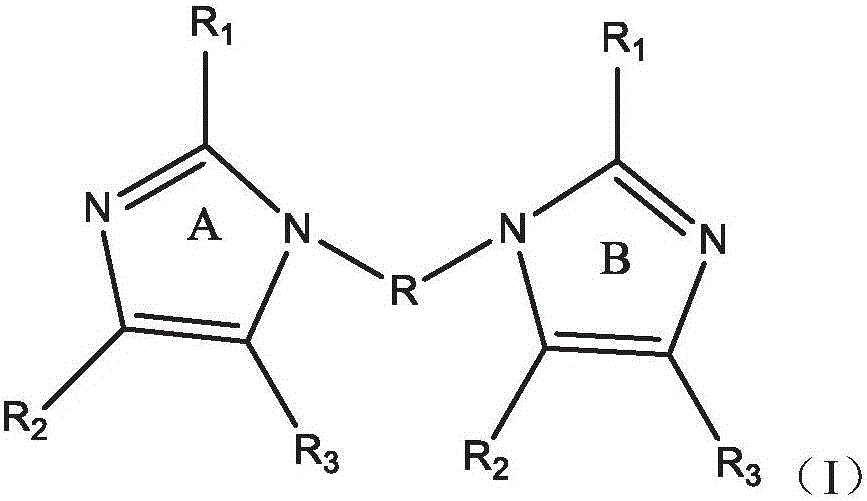
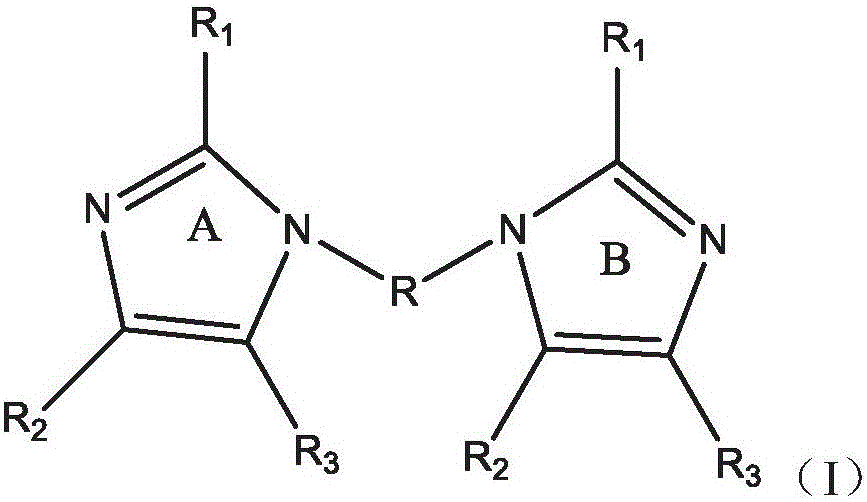
Polymers containing benzimidazole moieties as levelers
一种聚合物、化合物的技术,应用在有机化学、印刷电路制造、印刷电路等方向,能够解决损害电镀浴均镀能力等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0090] Example 1 (comparison)
[0091] Imidazole (6.8 g, 100 mmol) and sodium hydride (2.64 g, 11 mmol) were dissolved in 50 mL of anhydrous tetrahydrofuran. The mixture was stirred and cooled in an ice bath for 2 hours until hydrogen evolution ceased. 1,4-Dibromobutane (10.8 g, 50 mmol) was added and the mixture was stirred at room temperature for 12 hours. After removing the precipitate, the solvent was removed to obtain 1,4-bis(N-imidazolyl)butane. By 1HNMR (Bruker (Bruker), 400MHz, H 2 O-d2) Analysis of 1,4-bis(N-imidazolyl)butane showed the following peaks, confirming the structure: δppm: 8.81 (s, 2H, Haram); 7.51 (m, 4H, Haram.); 4.21 (m, 4H, 2xCH 2 -N), 4.257(m, 4H, 2xCH 2 -NH) and 1.87-1.90 (m, 4H, 2xCH 2 -CH 2 -NH).
[0092] 1,4-Bis(N-imidazolyl)butane (2.18g, 10mmol) and 1,4-dibromobutane (2.16g, 10mmol) were dissolved in dimethylformamide and the mixture was heated at 100°C 24 hours. The resulting precipitate (Product A) was collected by filtration and was...
example 2
[0094] Benzimidazole (11.8 g, 100 mmol) and sodium hydride (2.64 g, 11 mmol) were dissolved in 50 mL of anhydrous tetrahydrofuran. The mixture was stirred and cooled in an ice bath for 2 hours until hydrogen evolution ceased. 1,4-Dibromobutane (10.8 g, 50 mmol) was added and the mixture was stirred at room temperature for 12 hours. After removing the precipitate, the solvent was removed by evaporation to obtain 1,4-bis(N-benzimidazolyl)butane. By 1HNMR (Bruker (Bruker), 400MHz, H 2 O-d2) Analysis of 1,4-bis(N-imidazolyl)butane showed the following peaks, confirming the structure: δppm: 8.07 (s, 2H, Haram); 7.625 (m, 2H, Harom.); 7.447 (m, 2H, Harom), 7.238(m, 4H, Harom) 4.89(m, 4H, 2xCH 2 -N), 4.25(m, 4H, 2xCH 2 -NH) and 1.87(m, 4H, 2xCH 2 -CH 2 -NH).
[0095] 1,4-bis(N-benzimidazolyl)butane (1.16g, 4mmol), 1,4-bis(N-imidazolyl)butane (1.14g, 6mmol) and 1,4-dibromobutane The alkane was dissolved in dimethylformamide and the mixture was heated at 100 °C for 24 hours. T...
example 3
[0097] Benzimidazole (4.72 g, 4 mmol), imidazole (4.08 g, 6 mmol) and sodium hydride (2.64 g, 11 mmol) were dissolved in 50 mL of anhydrous tetrahydrofuran. The mixture was stirred and cooled in an ice bath for 2 hours until hydrogen evolution ceased. 1,4-Dibromobutane (10.8 g, 50 mmol) was added and the mixture was stirred at room temperature for 12 hours. After removing the precipitate by filtration, 50 ml of dimethylformamide and 10.8 g of 1,4-dibromobutane (50 mmol) were added. The mixture was heated at 100°C for 24 hours. The resulting precipitate (product 2) was collected by filtration and washed with ethyl acetate. According to the NMR results, the molar ratio of benzimidazolium to imidazolium in the product was 1.27. Mn was found to be 3,407 and Mw was found to be 12,581.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com