Method for selective preparation of p-xylene and toluene from p-methylcyclohexene carboxaldehyde
A technology of methylcyclohexene formaldehyde and p-xylene, which is applied in chemical instruments and methods, production of hydrocarbons, and bulk chemicals, etc., can solve the problems of high energy consumption and long process routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
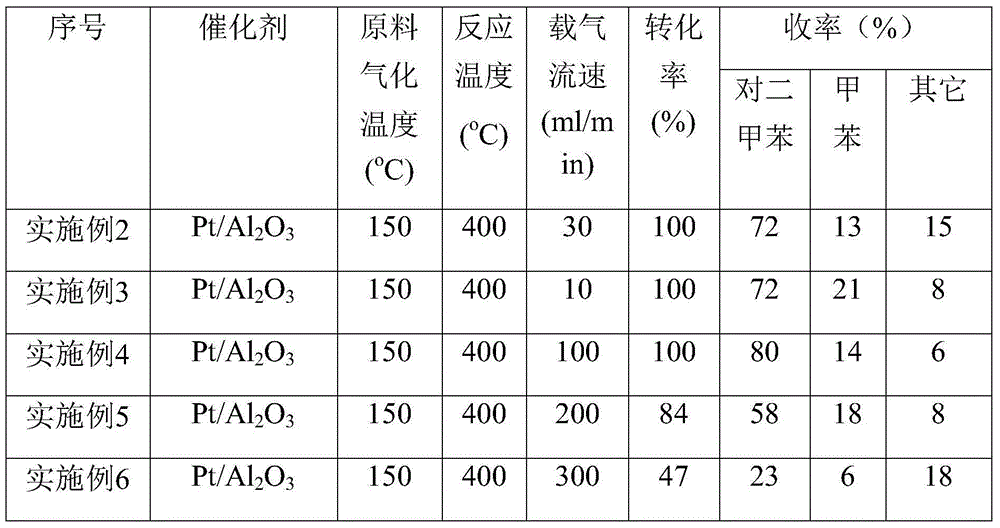
[0023] Preparation of supported catalysts: All supported catalysts were prepared by equal-volume impregnation method, with Pt / Al 2 o 3 As an example, the preparation process is as follows: Weigh 1.59g of chloroplatinic acid solution containing Pt3.767wt%, add water to dilute to 3.6g, and 5.94g of alumina (ground to 60-80 mesh, specific surface area> 280m 2 / g, pore volume > 0.37m 3 / g, bulk density 710kg / m 3 ) was immersed in the solution, dried at 80°C for 1 hour, dried at 120°C overnight, calcined at 500°C in air for 4 hours, cooled to room temperature and reduced with hydrogen at 300°C (60ml / min / g). Room temperature, O 2 / N 2 Mixed gas (O 2 Volume content 1%) in passivation 4h, obtain 1wt% / Al 2 o 3 , to collect the catalyst for later use.
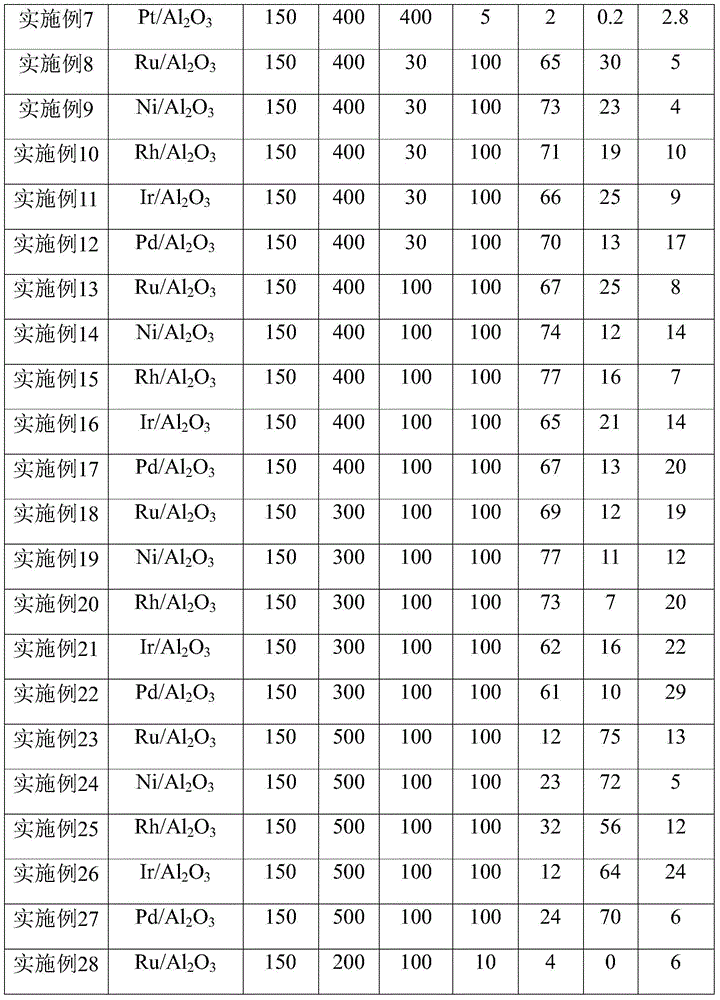
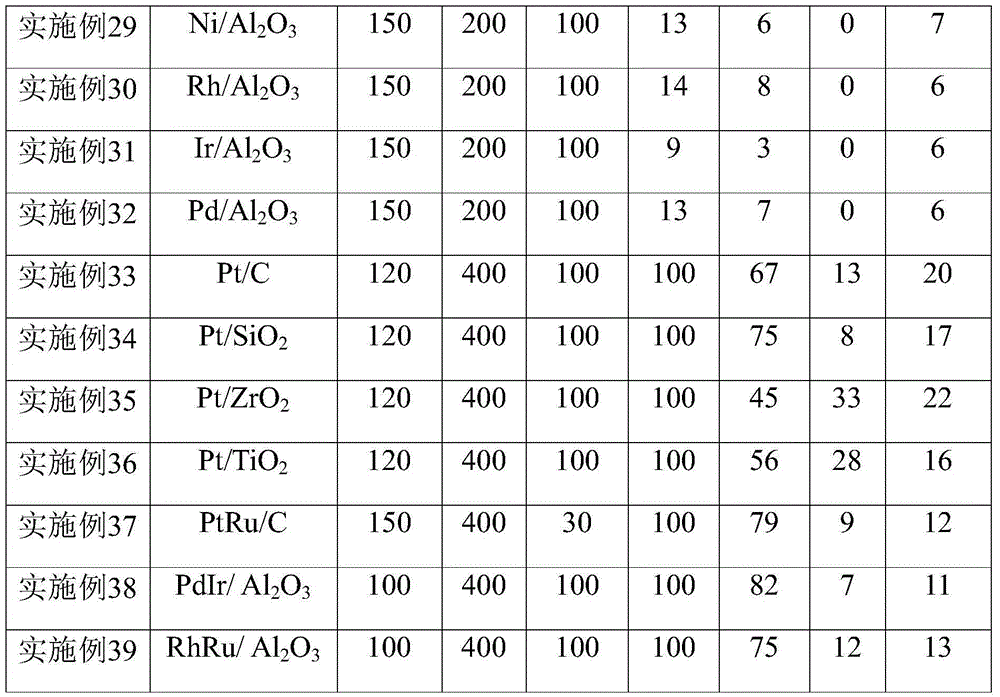
[0024]According to the above method, Pt, Ru, Ir, Rh, Pd, Ni catalysts loaded on activated carbon, alumina, silica, zirconia, and titania were prepared respectively, and the loading amount was 1 wt%.
[0025] The bimetallic catal...
Embodiment 2
[0027] 1wt% Pt / Al is filled in the tubular reactor (inner diameter 10mm) 2 o 3 (60-80 mesh) solid catalyst 1.0g, heated to 400 ° C, with N 2 The carrier gas was purged for half an hour to remove water, then 5.0 g of the reaction raw material was added to the raw material bottle, the temperature was raised to 150 ° C, and the 2 The carrier gas is used to introduce the reaction raw materials into the tubular reactor to ensure their circulation in the catalyst layer for reaction. The carrier gas flow rate is controlled at 30ml / min. The tail of the reactor receives a collecting bottle, which is cooled by liquid nitrogen to ensure that the product is completely collected. Weigh at last, calculate conversion rate and product yield quantitatively in conjunction with GC-MS, and reaction result is listed in Table 1.
Embodiment 3-7
[0029] Other reaction conditions were the same as in Example 2, and the carrier gas flow rate was controlled to be 10ml / min, 100ml / min, 200ml / min, 300ml / min, 400ml / min respectively. The reaction results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com