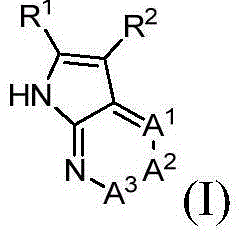
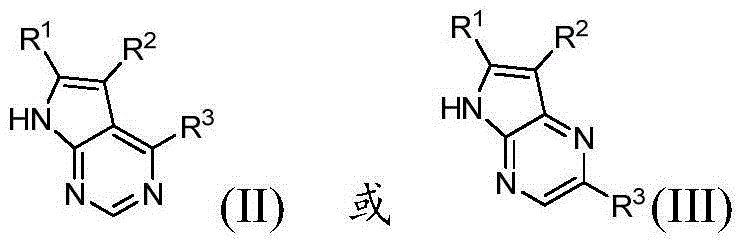
Azaindole derivatives, preparation method and applications thereof in medicine
A compound, heterocyclic group technology, applied in the field of azaindole derivatives, its preparation and its application in medicine, can solve the problems of increasing the risk of cancer and heart failure, seriousness, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] N-(1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-3-yl)acrylamide
[0080]
[0081] first step
[0082] tert-Butyl 1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-3-ylcarbamate
[0083] Compound 4-chloro-7H-pyrrolo[2,3-d]pyrimidine 1a (154mg, 1.0mmol), tert-butylpiperidin-3-ylcarbamate 1b (200mg, 1.0mmol), N, N -Diisopropylethylamine (387mg, 3.0mmol) and 1,4-dioxane (5mL) were mixed, and heated to 120° C. for 0.5 hour in microwave to react. Cool to room temperature and desolvate under reduced pressure. The residue was added with water (20 mL), extracted with ethyl acetate (20 mL×3). The combined organic phases were washed with saturated brine (20mL×3), dried over anhydrous sodium sulfate, filtered to remove the desiccant, precipitated under reduced pressure, and the residue was purified by silica gel column chromatography (dichloromethane / methanol=30 / 1). The target product tert-butyl 1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-3-ylcarbamate 1c (210 mg, white solid) w...
Embodiment 2
[0095] N-(1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-yl)acryloylamide
[0096]
[0097] Example 2 was synthesized with reference to the operation steps of Example 1, but in the first step, tert-butylpiperidin-4-ylcarbamate was used to replace tert-butylpiperidin-3-ylcarbamate.
[0098] MSm / z(ESI):272[M+1]
[0099] 1 HNMR(400MHz,DMSO-d6)δ11.68(s,1H),8.14(s,1H),8.05(d,J=7.8Hz,1H),7.18(s,1H),6.59(s,1H), 6.19(dd, J=17.2,10.0Hz,1H),6.09(d,J=18.9Hz,1H),5.58(d,J=11.9Hz,1H),4.58(d,J=13.2Hz,2H), 4.05-3.92 (m, 1H), 3.27-3.22 (m, 2H), 1.88 (d, J=10.0Hz, 2H), 1.45-1.37 (m, 2H).
Embodiment 3
[0101] 1-(3-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-1-yl)prop-2-en-1-one
[0102]
[0103] first step
[0104] tert-butyl 5-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-3,4-dihydropyridine-1(2H)-carboxylate
[0105] The mixture 4-chloro-7H-pyrrolo[2,3-d]pyrimidine 1a (1.0g, 6.5mmol), tert-butyl 5-(4,4,5,5-tetramethyl-1,3,2 -Dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxylate 3a (1.0 g, 6.5 mmol), sodium carbonate (1.4 g, 13.0 mmol), PdCl 2 (dppf) (475mg, 0.65mmol), 1,4-dioxane (20mL) and water (10mL) were heated to 95°C and stirred overnight under nitrogen protection. After the reaction solution was cooled to room temperature, water (50 mL) was added and extracted with ethyl acetate (30 mL×3). The organic phase was dried with anhydrous sodium sulfate, the desiccant was removed by filtration, and the solvent was precipitated under reduced pressure. The residue was purified by silica gel column chromatography (dichloromethane / methanol=50 / 1) to obtain the target product tert-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com