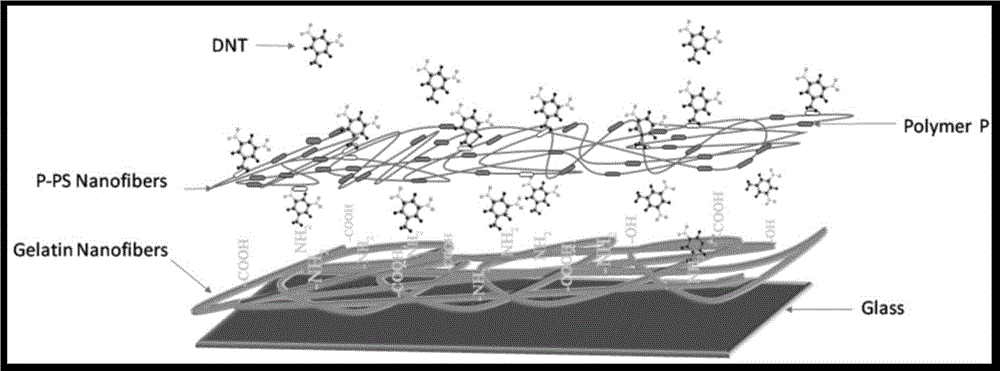
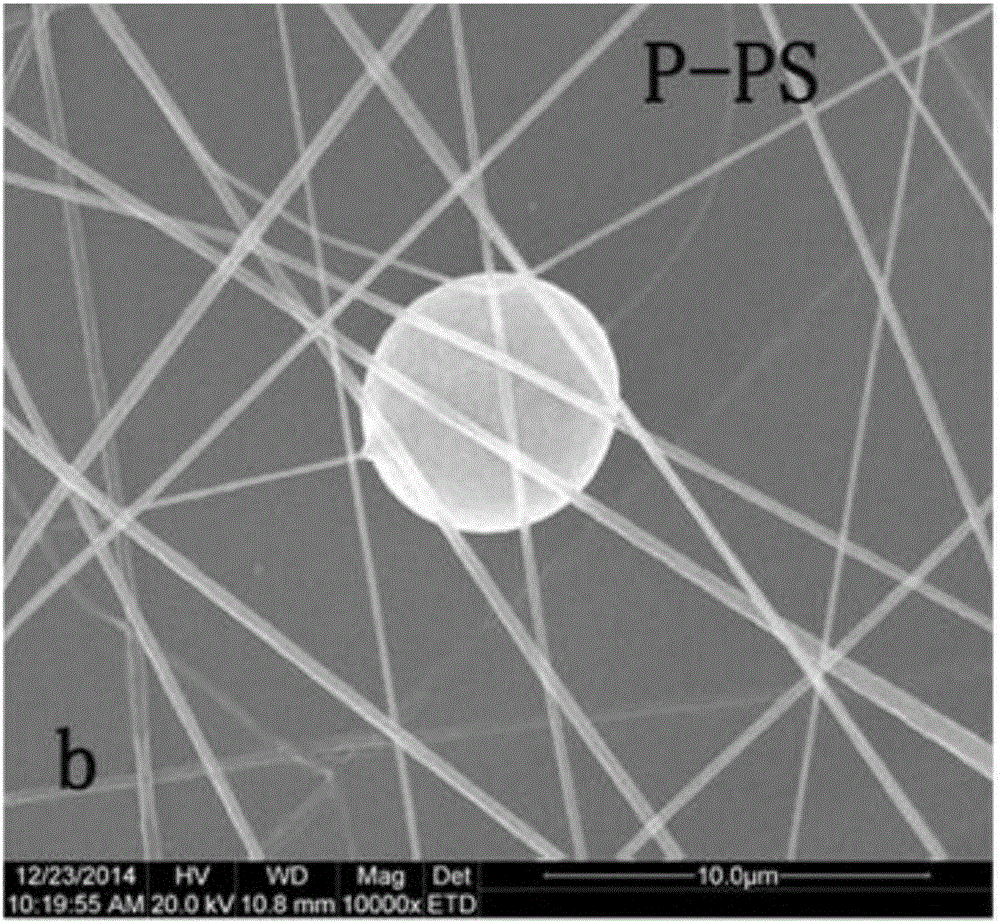
Fluorescent sensing polymer material for detecting nitryl explosives and preparation method
A polymer material and fluorescent sensing technology, which is applied in the field of fluorescent sensing, can solve problems affecting sensing performance, polymer self-quenching, affecting fluorescence intensity, polymer solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] A synthetic method for fluorescent sensing polymer P, comprising the following steps:
[0068] 2,5-dibromothiophene (200mg, 0.9mmol), polyphenylene vinylene polymer (376mg, 0.9mmol) and 2,7-dibromo-9,9-diphenylfluorene (426.6mg, 0.9mol) Dissolve in anhydrous diisopropylamine (DIPA, 2mL) and anhydrous toluene (30mL), place in a 50ml three-necked flask. Pass through argon for 30 minutes, add PdCl 2 (PPh 3 ) 2 (36mg, 2.6×10 -2 mmol), PPh 3 (270mg, 0.90mmol) and CuI (40.5mg, 0.3mmol). 100 ° C reflux reaction for 24h. Then, according to the purification, the fluorescent sensor polymer P is obtained, and the structural formula is shown in Formula 1 of the present invention.
[0069] Fluorescent sensor polymer P: dark yellow solid (400mg, 69%). 1 HNMR: (CDCl 3 ,400MHz), δ(ppm):0.0-2.0(m,20H),7.0-7.2(m,3H),7.4(d,1H,J=7.2HZ),6.0(d,3H,J=5.5HZ) ,7.6-7.9(m,2H).FT-IR(KBr):3060,2967,2921,2850,2198,1710,1600,1452,1414,1259,1103,1026,886,823,753,692,513cm -1 Calcdfor(C 58 h ...
Embodiment 2
[0070] Embodiment 2 A synthetic method of fluorescent sensing polymer P, comprising the following steps:
[0071] 2,5-dibromothiophene (210mg), polyphenylene vinylene polymer (378mg) and 2,7-dibromo-9,9-diphenylfluorene (438mg) were dissolved in anhydrous diisopropylamine (DIPA, 2.5mL) and anhydrous toluene (22mL), placed in a 50ml three-necked flask. Argon protection was passed through for 40 minutes, and PdCl was added 2 (PPh 3 ) 2 (21mg), PPh 3 (252mg) and CuI (63mg). 90 ° C reflux reaction for 36h. Then, according to the purification, the fluorescent sensor polymer P is obtained, and the structural formula is shown in Formula 1 of the present invention.
Embodiment 3
[0073] Preparation of electrospun PS-P film: fluorescent sensing polymer P (0.4 mg) (polymer in Example 1) and PS (0.4 g) were dissolved in 4 ml mixed solution (DMF:THF=3:1) , and stirred for 24 hours. Transfer the resulting solution to the syringe of the electrospinning device. Electrospinning was performed at 20 kV for an acceptance distance of 25 cm. The flow rate of the solution was 1mLH via a syringe pump -1 Control at a constant rate. Electrospun nanofibrous membranes (1.0 cm x 1.7 cm) were formed on glass slides, which were attached to metal plate collectors on aluminum plates. The electrospun nanofibrous membrane (20 μm) was oven dried at 30 °C for 10 h to remove residual organic solvent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com