Method for in-vitro expansion of CD8<+>T cells
An in vitro expansion and cell technology, which is applied in the direction of blood/immune system cells, animal cells, vertebrate cells, etc., can solve the problems of T cell proliferation and killing function inhibition, so as to promote the anti-tumor effect, increase the expansion multiple, Inhibition of negative regulatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 (experimental group):
[0028] (1) Obtain a mixture of tumor cells and TIL cells:
[0029] Take 500ml of pleural fluid from the patient and inject it into an anticoagulant bottle containing heparin sodium, shake it gently to mix it evenly, divide it evenly into centrifuge tubes, and centrifuge at 2000r / min for 10min. After centrifugation, discard the supernatant. Wash 2 times with saline.
[0030] (2) Separation of TIL cells:
[0031] Adhesion method: Take 15ml of the cells obtained in step (1) and add an equal volume of normal saline to 30ml (this is right), mix well, add 15ml of 100% lymphocyte separation solution to the centrifuge tube, the lymph separation solution and the cells The volume ratio of the suspension is 1:2, slowly superimpose 30ml of the cell suspension on the lymph separation medium along the tube wall, centrifuge at 2000r / min for 20min, collect the buffy coat after centrifugation, which is the mixture of tumor cells and TIL cells, 1500...
Embodiment 2
[0036] Example 2: No anti-PD-1 monoclonal antibody added (control group)
[0037] Except for (3) cell expansion, no anti-PD-1 monoclonal antibody was added, and other specific operation steps were as in Example 1.
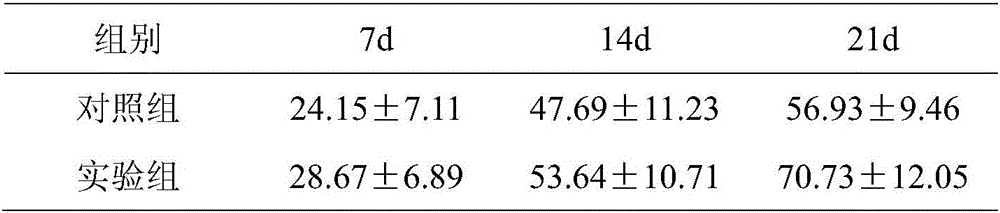
[0038] The TIL cells obtained from the experimental group of Example 1 and the control group of Example 2 were detected by amplification factor
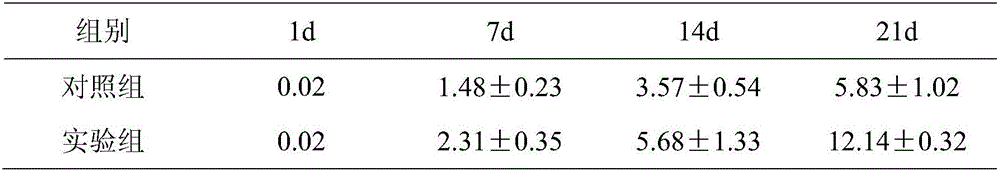
[0039] On the 1st, 7th, 14th, and 21st days, 200 μl of cells were taken, blown into single cells, and counted by a Counterstar automatic blood cell counter. Cell expansion factor = total number of cells counted on that day / total number of cells before culture on the first day. The experimental results are shown in Table 1.
[0040] Table 1 The change of cell number of TIL cells at different times (×10 8 / L)
[0041]
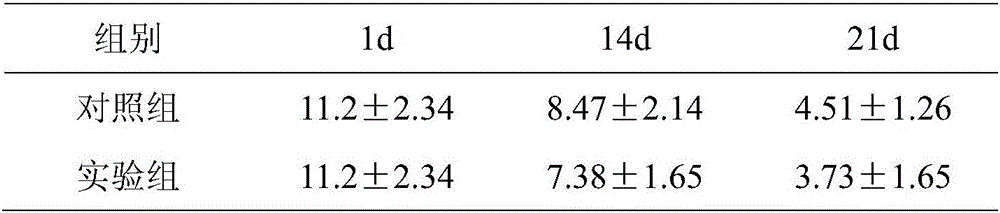
[0042] Treg phenotype detection was performed on the TIL cells obtained from the experimental group of Example 1 and the control group of Example 2
[0043] Collect 2×10 on days 1, 7, 14, 21 6 / ml cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com