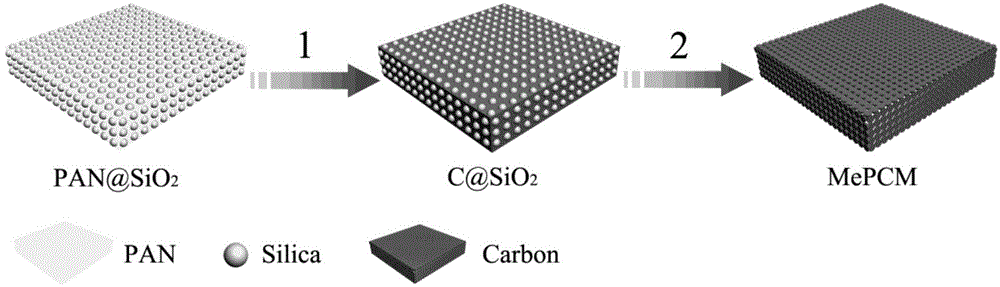
Porous carbon membrane for lithium-sulfur batteries and application of porous carbon membrane
A lithium-sulfur battery and porous carbon technology, which is applied in the direction of lithium batteries, battery electrodes, non-aqueous electrolyte batteries, etc., can solve the problems of poor conductivity and reduce the proportion of effective materials in electrodes, and achieve good comprehensive performance. The preparation process is simple and mature, The effect of improving the utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
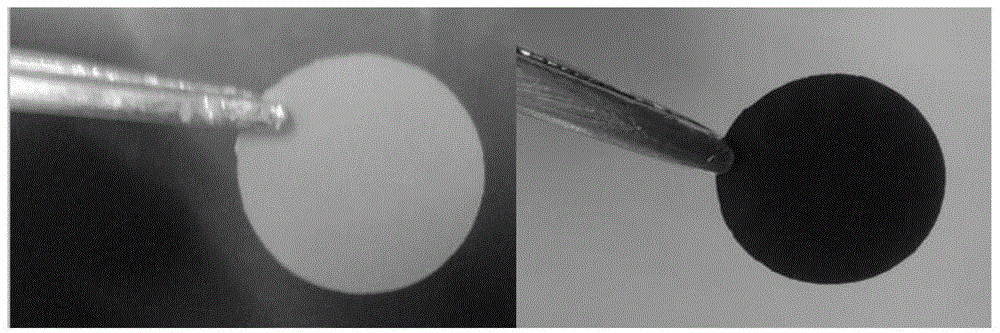
[0056] Weigh 1.0g of polyacrylonitrile (PAN, Mw=150000) and 0.1g of polyvinylpyrrolidone (PVP, Mw=10000), stir and dissolve in a solution of 15.4g of N,N-dimethylformamide, add 1.0g of hydrophobic Silica particles (diameter d = 20nm), after stirring for 24 hours, paved into a film (scraper adjustment 500μm), placed on a 70 ℃ drying table, dried overnight, and cut into small discs with a diameter of 14mm (such as figure 2 (shown on the left), placed in a muffle furnace for pre-oxidation at 250°C, with a heating rate of 1°C min -1 , keep the temperature for 4 hours, after cooling to room temperature, put it in a tube furnace for carbonization at 900-1200 degrees Celsius, and the heating rate is 5℃min -1 , constant temperature for 4h, after cooling to room temperature, carbonized small discs (such as figure 2 (shown on the right) placed in 20wt% HF to etch the template for 48 hours, washed with deionized water several times and dried. Sulphur-filled and assembled battery test...
Embodiment 2
[0059] Weigh 0.67g polyacrylonitrile (PAN, Mw=150000), 0.67g PMMA, and 0.1g polyvinylpyrrolidone (PVP, Mw=10000), stir and dissolve in a solution of 15.4g N,N-dimethylformamide, Add 0.67g of hydrophobic silica particles (diameter d=20nm), and stir for 24 hours to obtain a mixed solution. Subsequent film laying, sulfur filling, and battery assembly test steps are the same as in Example 1.
[0060] The specific capacity of the first cycle discharge is 1255mAhg -1 , the capacity is maintained at 984mAhg after 20 cycles -1 , The capacity retention rate was 78.4%.
Embodiment 3
[0062] Weigh 0.8g of polyacrylonitrile (PAN, Mw=150000) and 0.1g of polyvinylpyrrolidone (PVP, Mw=10000), stir and dissolve in 15.4g of N,N-dimethylformamide solution, add 1.2g of carbonic acid Calcium particles (diameter d=20nm), after stirring for 24 hours, a mixed solution was obtained. Subsequent film laying, sulfur filling, and battery assembly test steps are the same as in Example 1.
[0063] The specific capacity of the first cycle discharge is 1320mAhg -1 , the capacity is maintained at 1088mAhg after 20 cycles -1 , The capacity retention rate was 82.4%.
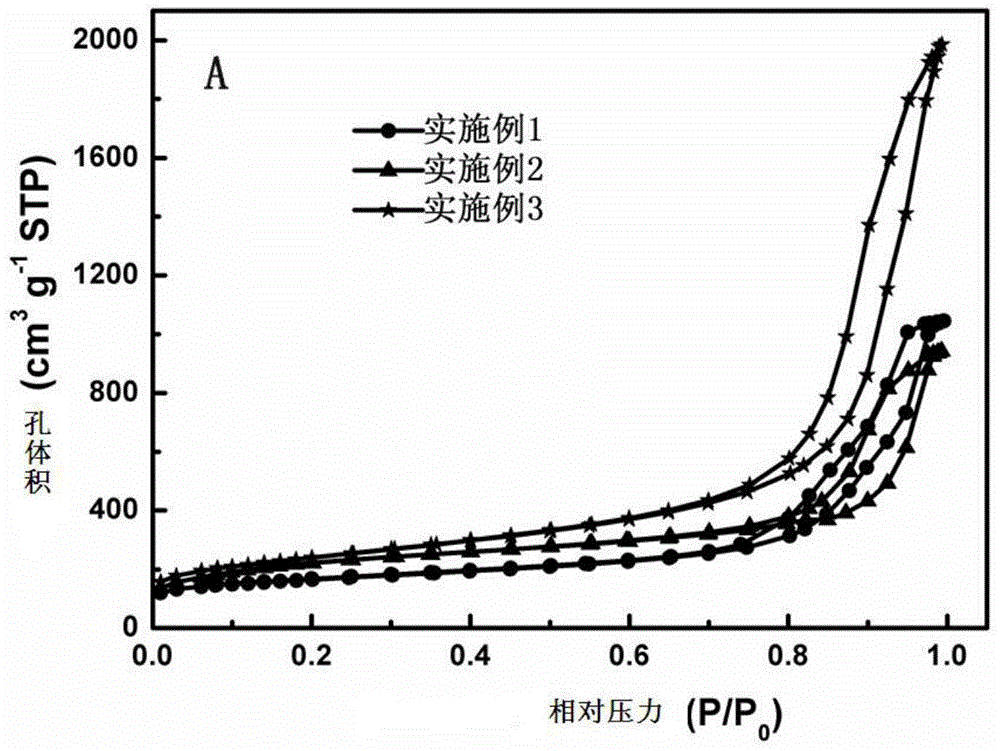
[0064] Depend on figure 2 It can be seen that the morphology of this type of carbon film has no obvious change before and after carbonization, and the overall size is slightly reduced, which is caused by the volume shrinkage of the polymer during carbonization; image 3 It can be seen that Example 1 is a single mesoporous distribution, while Examples 2 and 3 are micro-mesoporous bimodal pore structure distributi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com