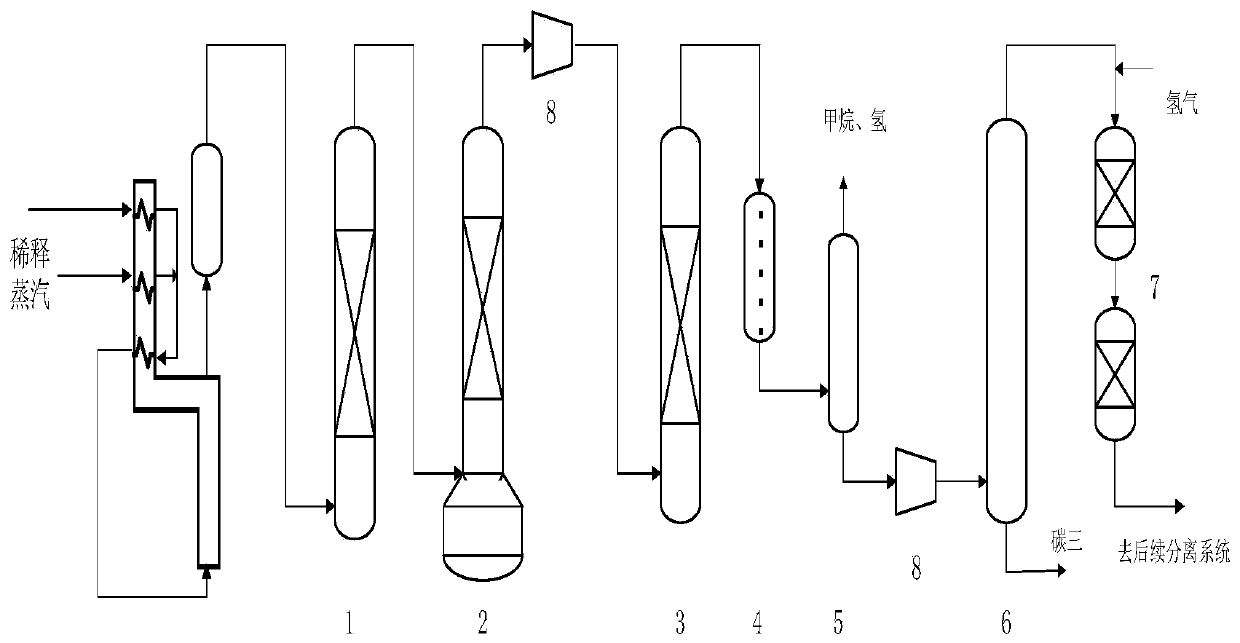
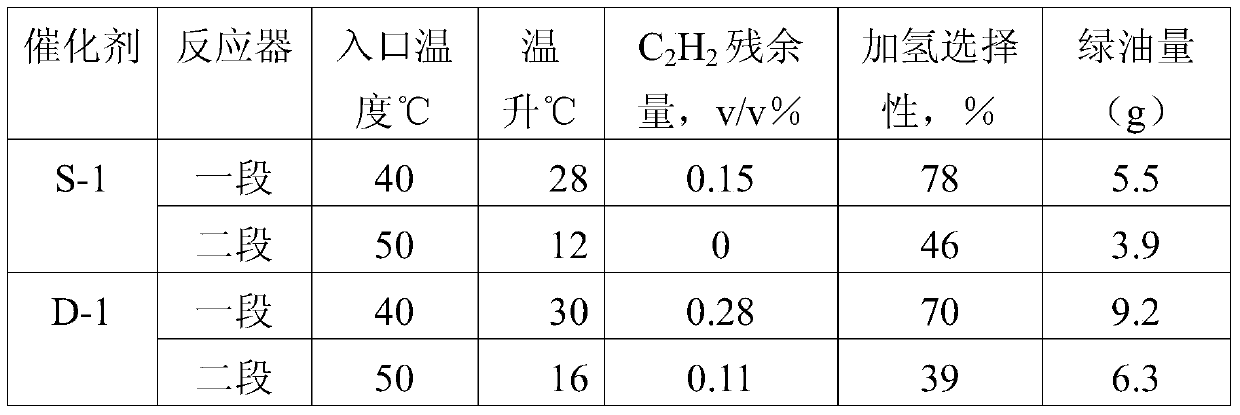
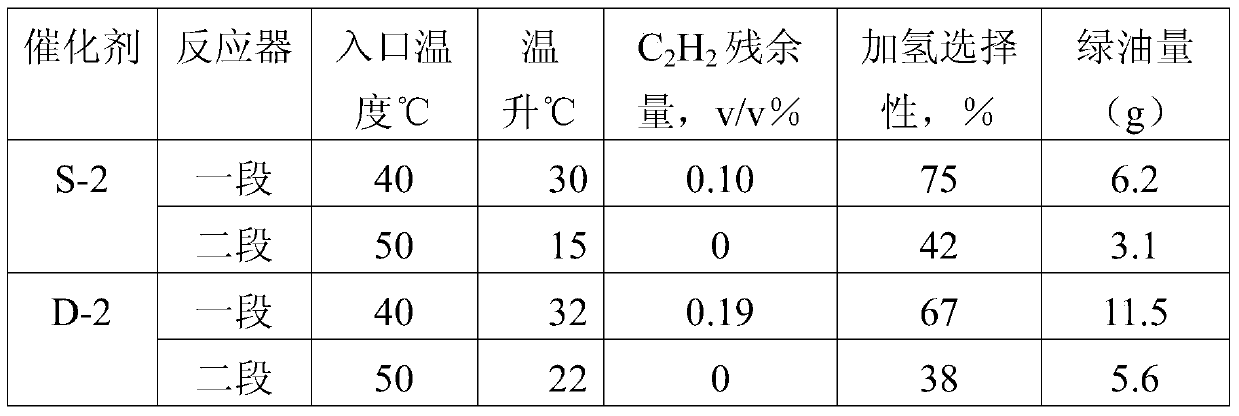
A method for selective hydrogenation of carbon 2 in a sequential separation process
A technology of selective hydrogenation and sequential separation, applied in chemical instruments and methods, educts, hydrogenation to hydrocarbons, etc., can solve problems such as weak complexation, increased catalyst cost, and insufficient loading of active components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Weigh Φ3.5, the specific surface area is 20.0m 2 / g, pore volume 0.31mL / g, bulk density 0.82g / ml spherical α-Al 2 o 3 Carrier 500g.
[0047] Dissolve 34.12g of 4,4-dihydroxy-2,2-bipyridine in 650mL ethanol solution, impregnate the above-mentioned carrier in the above-mentioned solution, and let the 4,4-dihydroxy-2,2-bipyridine completely After being loaded on the alumina support, it was dried at 60°C for 10 h to obtain the hydroxyl-bipyridine / Al 2 o 3 Prebody.
[0048] Weigh 0.37g Pd(NO 3 ) 2 , 0.79g AgNO 3 , dissolved in 600mL of deionized water, added 10ml of nitric acid and stirred until completely dissolved, adjusted the pH value to 3.5, and prepared a mixed solution. The above-mentioned hydroxyl-bipyridine / Al 2 o 3 The precursor was added to the prepared solution, stirred for 10 minutes, left to stand for 2 hours, and the residue was poured out to obtain PdAg-hydroxyl-bipyridine / Al 2 o 3 Precursor (number of moles of hydroxyl-bipyridine: (Pd+Ag)=30). A...
Embodiment 2
[0065] Weigh Φ4.0mm, height 4.0mm, specific surface area is 55.0m 2 / g, cylindrical θ-Al with a pore volume of 0.45ml / g and a bulk density of 0.68g / ml 2 o 3 Carrier 500g.
[0066] Dissolve 1.4g of 4,4-dihydroxy-2,2-bipyridine in 600mL ethanol solution, impregnate the above-mentioned carrier in the above-mentioned solution, and let the dihydroxy-2,2-bipyridine be completely loaded on the alumina after standing for 8 hours After being mounted on the carrier, dry at 90°C for 8 hours to obtain hydroxy-bipyridine / Al2 o 3 Prebody.
[0067] Weigh 0.73gPd(NO 3 ) 2 , 2.37gAgNO 3 , dissolved in 600mL deionized water, add 10ml nitric acid and stir until completely dissolved, adjust the pH to 2.5, and make a mixed solution. The above-mentioned hydroxyl-bipyridine / Al 2 o 3 Add the precursor to the prepared solution, stir for 60 minutes, let it stand for 8 hours, pour out the residual liquid, and dry the remaining solid at 110°C for 6 hours to obtain PdAg-hydroxy-bipyridine / Al 2 o...
Embodiment 3
[0087] Weighing Φ3.0mm, the specific surface area is 35.0m 2 / g, the pore volume is 0.20ml / g, the heap ratio is 0.75g / ml toothed spherical carrier 500g, wherein Al 2 o 3 460g, titanium oxide 40g, Al 2 o 3 It is a mixed crystal form of θ and α. Dissolve 71.07g of 6,6'-dihydroxy-3,3'-bipyridine in 650mL ethanol solution, impregnate the above carrier in the above solution, and let 6,6'-dihydroxy-3,3' -Bipyridine was completely loaded on the alumina support, and dried at 120°C for 4h to obtain hydroxy-bipyridine / Al 2 o 3 Prebody.
[0088] Weigh 0.40gPd(NO 3 ) 2 ,0.39gAgNO 3 , dissolved in 550mL deionized water, add 10ml nitric acid and stir until completely dissolved, adjust the pH value to 3, and make a mixed solution. The above-mentioned hydroxyl-bipyridine / Al 2 o 3 Add the precursor to the prepared solution, stir for 60 minutes, let stand for 12 hours, pour out the residue, and dry at 100°C for 8 hours to obtain PdAg-hydroxy-bipyridine / Al 2 o 3 Precursor (number o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com