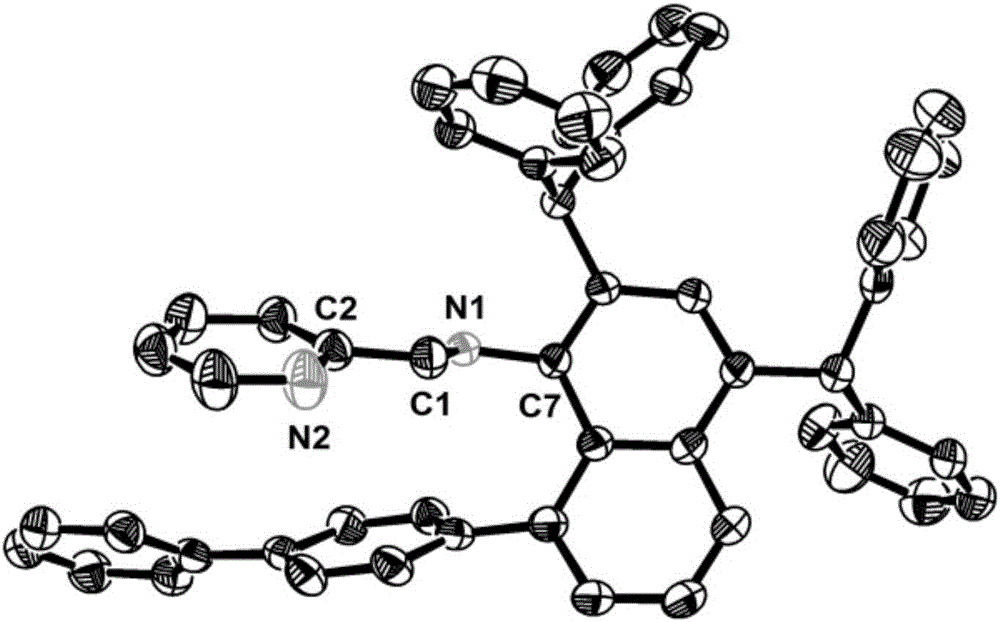
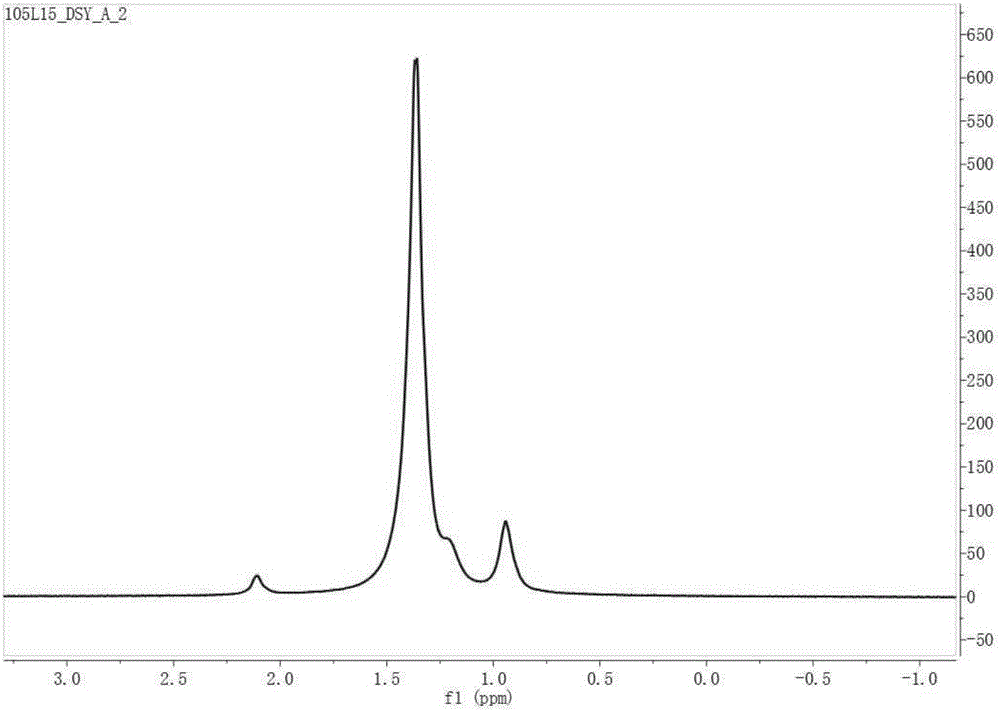
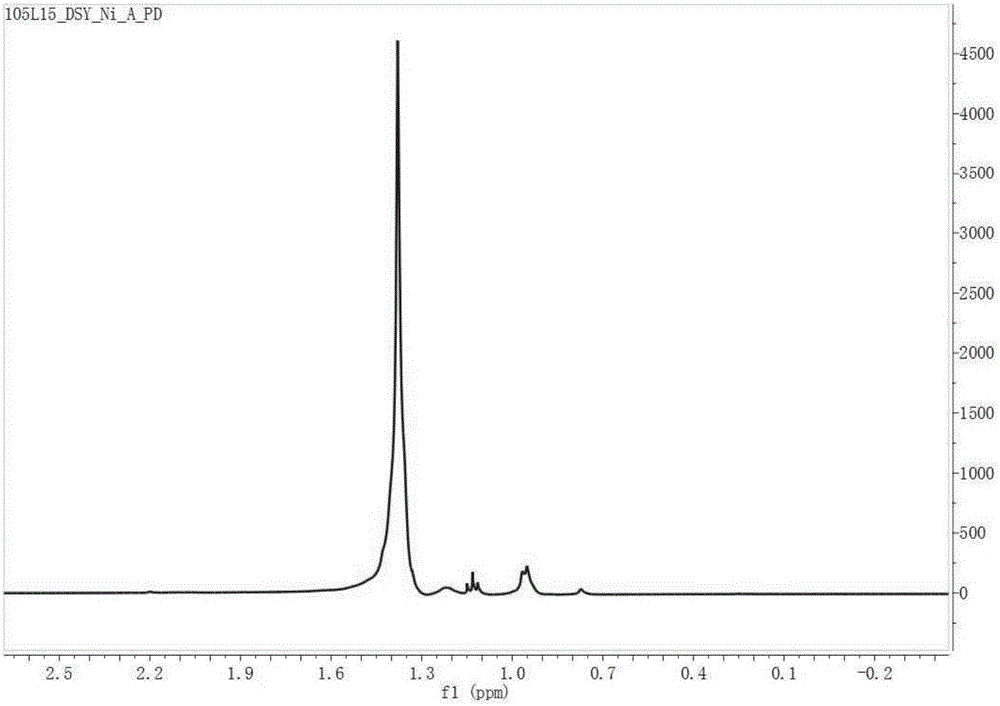
Pyridine imine compound and preparation method thereof, pyridine imine nickel catalyst and preparation method thereof and polyolefin
A pyridine imine nickel and pyridine imine technology, applied in the field of catalysts, can solve the problems of reducing the molecular weight, unable to increase the molecular weight of polyethylene, unable to effectively increase the molecular weight of polyethylene and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The present invention also provides a preparation method of the above-mentioned pyridine imine compounds, comprising:
[0055] The substituted naphthylamine derivative represented by formula (I-1) is reacted with the pyridine carbonyl compound represented by formula (I-2) to obtain the pyridine imine compound represented by formula (I).
[0056]
[0057] where the R 1 , R 2 with R 3 Each is independently hydrogen, aryl or arylalkyl; the number of carbon atoms of the alkyl in the arylalkyl is 1-6;
[0058] The R 4 , R 5 with R 6 each independently hydrogen, C1-C6 alkyl, nitro, C1-C6 alkoxy, N,N-dialkylamino or trifluoromethyl; in the N,N-dialkylamino The number of carbon atoms in the alkyl group is 1-6.
[0059] where the R 1 , R 2 , R 3 , R 4 , R 5 with R 6 All are the same as above, and will not be repeated here.
[0060] In the present invention, the substituted naphthylamine derivatives represented by the formula (I-1) are preferably prepared accordi...
Embodiment 1
[0116] Example 1: 2,4-bis(benzhydryl)-8-phenyl-1-naphthylamine
[0117]
[0118] Add 20 millimoles of 8-phenyl-1-naphthylamine and 40 millimoles of diphenylmethanol to a 150-ml pressure-resistant flask, heat to 120 degrees, and then add 10 millimoles of concentrated hydrochloric acid solution of anhydrous zinc chloride. Mole, the reaction bubbles, heated to 160 degrees; after half an hour of reaction at 160 degrees, stop the reaction, cool to room temperature, dissolve in 200 ml of dichloromethane solution, wash with 3 times 100 ml of water, dry over anhydrous magnesium sulfate . Filter, concentrate to 20 mL, add 200 mL of methanol to the product, wash with 3 times 100 mL of methanol to give a white powder solid as 2,4-bis(benzhydryl)-8-phenyl-1-naphthalene The amine was vacuum-dried to obtain 10.49 g of a solid with a yield of 95% and a purity greater than 99%.
[0119] Utilize nuclear magnetic resonance to analyze the 2,4-bis(benzhydryl)-8-phenyl-1-naphthylamine obtaine...
Embodiment 2
[0121] Example 2: 2,4-bis(benzhydryl)-8-p-methylphenyl-1-naphthylamine
[0122]
[0123] Add 20 mmoles of 8-p-methylphenyl-1-naphthylamine and 40 mmoles of diphenylmethanol in a 150 ml pressure-resistant flask, heat to 120 degrees, then add concentrated hydrochloric acid of anhydrous zinc chloride The solution was 10 millimoles, the reaction bubbled, heated to 160 degrees, and after half an hour of reaction at 160 degrees, the reaction was stopped, cooled to room temperature, and dissolved in 200 milliliters of dichloromethane solution. Wash with 3 times 100 ml of water and dry over anhydrous magnesium sulfate. Filter, concentrate to 20 mL, add 200 mL of methanol to the product, wash with 3 times 100 mL of methanol to give a white powder solid as 2,4-bis(benzhydryl)-8-p-methylphenyl- 1-Naphthylamine was vacuum-dried to obtain 10.75 g of solid with a yield of 95% and a purity of more than 99%.
[0124] The 2,4-bis(benzhydryl)-8-p-methylphenyl-1-naphthylamine obtained in Ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com