An inactivated Zika virus vaccine
A Zika virus and vaccine technology, applied in the field of biopharmaceuticals, can solve the problems of blank culture process, purification process and preparation process, vaccine immunogenicity and stability need to be studied, etc., to avoid Brazilian microcephaly, reduce Operation steps, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of Cell Working Seed and Preparation of Virus Liquid
[0044] 1. Preparation of high-density cell working seeds
[0045] Use 199 culture medium to dilute Vero main cell seed dilution ratio to 60:1, inoculate to 175cm 2 Cells were cultured in square flasks at a temperature of 36.0°C±1°C. After the cells are fully covered with the cell bottle, continue passage for 4 generations according to the ratio of 1:4 to 1:6. The last generation was transferred to five 40-layer cell factories, trypsinized, and centrifuged to remove the supernatant. Use freezing solution containing 10% DMSO to resuspend, and the cell concentration after mixing is 2.5×10 7 pieces / ml. The cell seeds were subjected to gradient cooling in a temperature program cooling device, keeping the temperature down by 1°C per minute. After the temperature drops to -196°C, it is stored in liquid nitrogen, and the working cell seeds are valid for 10 years.
[0046] 2. Vero cell culture
...
Embodiment 2
[0051] Example 2 Screening of Medium
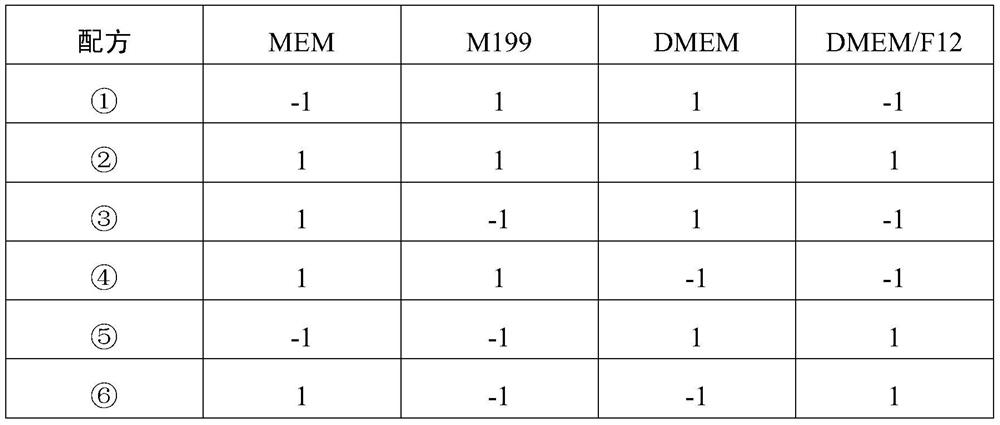
[0052] Select commercially available MEM, M199, DMEM, DMEM / F12 medium, use the DOE software of Minitab17 for experimental design, carry out virus culture according to the designed medium formula, compare the results of virus titer and virus immunogenicity, and choose the best Medium. The medium formula of the experimental design is shown in Table 1, wherein "1" represents adding the medium, and "-1" represents not adding the medium. If there are several "1" in the recipe, make several equal parts, and each medium is divided into one equal part. The results of virus titer and immunogenicity are shown in Table 2 and Table 3, wherein the medium in which M199 and DMEM / F12 are mixed in equal volume at 1:1 produces significantly higher virus immunogenicity and titer than other mediums.
[0053] Table 1 DOE test medium formula
[0054]
[0055]
[0056] Table 2 Virus titer results
[0057] formula Titer results (CCID 50 / m...
Embodiment 3
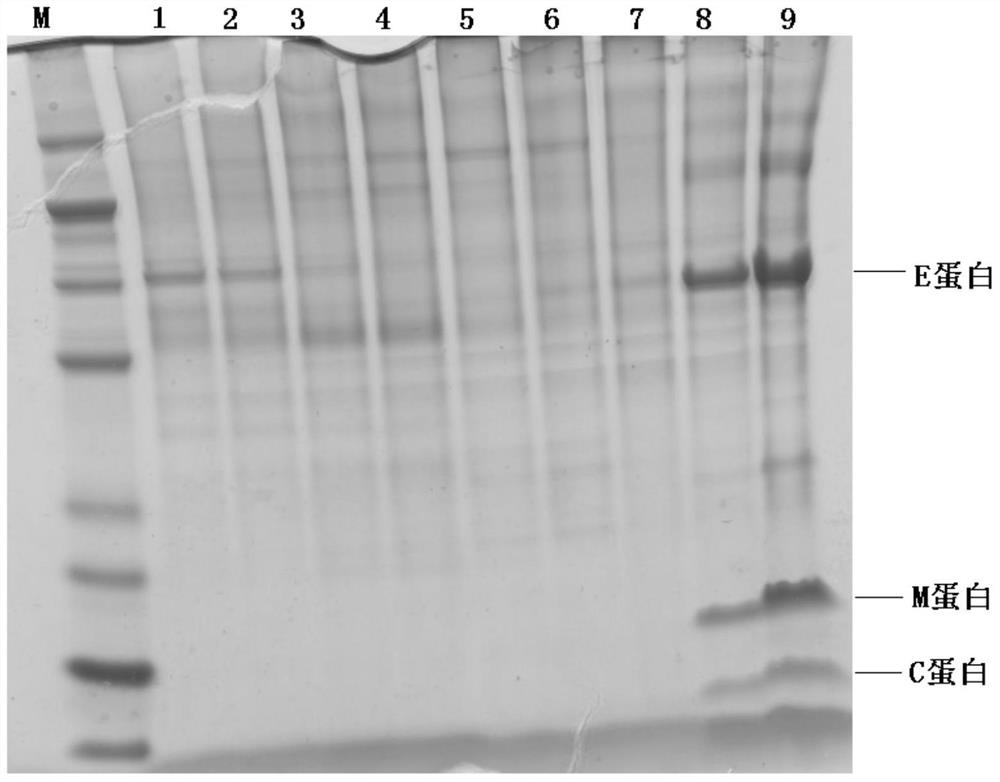
[0061] Example 3 Zika virus inactivation and purification
[0062] 1. Inactivation of Zika virus Add formaldehyde at a final concentration of 200 μg / ml to the Zika virus harvest solution, and inactivate it at 37°C for 7 days. That is, the inactivation of Zika virus is completed.
[0063] 2. Purification of Zika virus
[0064] (1) Clarification Filter the virus liquid through filter elements with continuous three-stage pore diameters of 3-0.8 μm, 0.8-0.65 μm, and 0.65-0.22 μm or centrifuge (including continuous flow centrifugation) with a speed of 2000-5000 rpm and a centrifugation time of 0.5-4 hours, to obtain virus clarification;
[0065] (2) Concentration The virus clarified liquid was concentrated by a Pall tangential flow membrane filtration system with a molecular weight cut-off of 300KD, and the concentration factor was 100 times.
[0066] (3) Density gradient centrifugation The low and high concentrations of the sucrose solution are 25% and 55% respectively. The de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com