Alkaloid compound with 1,2,3-triazole structure segment and application of compound
A technology of structural fragments and compounds, applied in the field of alkaloid compounds, can solve the problems of poor cell penetration ability and unsatisfactory cell level activity of peptide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
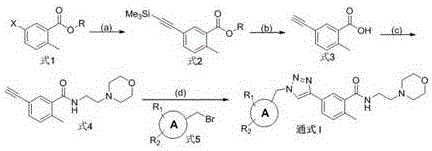
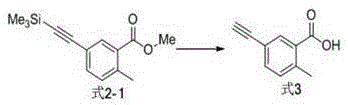
[0023] The preparation of formula 2-1 compound
[0024]
[0025] 5.0g formula 1-1 compound and 2.57g trimethylsilyl acetylene are dissolved in 25mLN, in the mixed solution of N-dimethylformamide and 5mL triethylamine, nitrogen protection, add 1.53g tetrakis (triphenylphosphine subsequently) ) palladium and 0.4g cuprous iodide, heated to 50 o C and stirred at this temperature for 6 hours. Afterwards, the reaction solution was concentrated, and the organic phase was extracted with ethyl acetate / water. The organic phase was washed successively with water and saturated brine, and concentrated. The residue was purified by column chromatography to obtain 3.90 g of compound Formula 2-1 as a colorless oil, with a yield of 73%. 1 HNMR (400MHz, CDCl 3 )δ7.84(1H,d,J=9Hz),7.34(1H,s),7.31(1H,d,J=9Hz),3.88(3H,s),2.56(3H,s),0.25,(9H ,s). 13 CNMR (100MHz, CDCl 3 )δ167.4, 140.2, 135.0, 130.5, 129.2, 129.0, 126.7, 104.1, 96.9, 51.9, 21.5, 0.2.
Embodiment 2
[0027] The preparation of formula 3 compound
[0028]
[0029]Dissolve 3.90g of the compound of formula 2-1 in 50mL of methanol, ice bath to 0 o C, add 2.48g potassium hydroxide. After the reaction was carried out for 2 hours, it was heated to reflux for 4 hours. After the reaction solution was concentrated under reduced pressure, 50 mL of water was added, and the aqueous phase was extracted three times with ethyl acetate. Afterwards, the aqueous phase was acidified to pH~3 with 10% hydrochloric acid solution, and extracted three times with ethyl acetate. The organic phase was washed with saturated sodium bicarbonate, saturated brine, and water successively, and dried over anhydrous magnesium sulfate. Finally, after filtration and concentration of the organic phase, 2.5 g of light yellow solid compound Formula 3 was obtained, with a yield of 98%. It can be directly used in the next reaction. 1 HNMR (400MHz, CDCl 3 )δ8.03(1H,d,J=8Hz),7.41(1H,s),7.39(1H,d,J=8Hz),3.22(1H,...
Embodiment 3
[0031] The preparation of formula 4 compound
[0032]
[0033] Dissolve 2.50g of the compound of formula 3 in 30mL of dichloromethane, ice bath to 0 o C, followed by the addition of 2.33 g HOBT, 4.1 mL N,N-diisopropylethylamine (DIEPA) and 3.28 g EDCI. After the reaction was carried out for 0.5 hour, 4.06 g and 2-morphine ethylamine were added. The reaction was stirred overnight, and the organic phase was washed successively with saturated brine and water, and dried over anhydrous magnesium sulfate. Filter and concentrate to obtain a residue. The residue was purified by column chromatography to obtain 3.60 g of white solid compound Formula 4 with a yield of 85%. 1 HNMR (400MHz, CDCl 3 )δ7.32(2H,d,J=12Hz),7.31(1H,s),6.41(1H,s),3.68(4H,dd,J=4,4Hz),3.51(2H,m),3.11( 1H,s),2.36(2H,t,J=4Hz),2.47(4H,m),2.41(3H,s). 13 CNMR (100MHz, CDCl 3 )δ169.2, 136.7, 136.1, 134.5, 129.4, 126.9, 123.6, 82.8, 78.3, 66.8, 56.9, 53.3, 35.9, 19.6. ESI-MS: 273.0 [M+H + ]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com