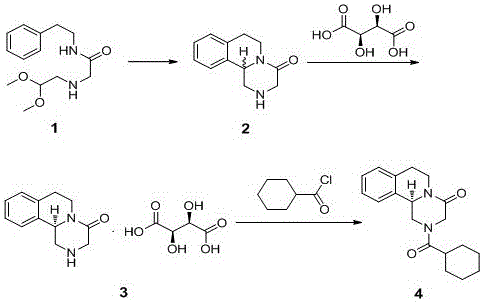
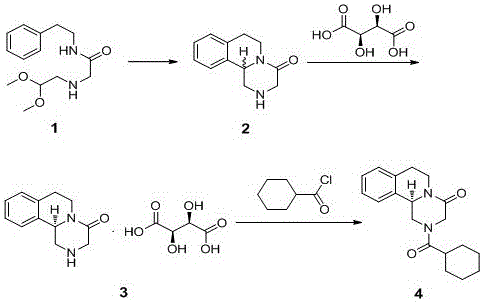
Process for synthesizing levopraziquantel
A technology of L-praziquantel and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of high production cost, many toxic reagent raw materials, poor product quality, etc., and achieves reduction of synthesis cost, simple and controllable operation method, and stable product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Add sulfuric acid (250g) dropwise to a solution of N-(2-phenyl)ethyl-2-{(acetal acetal)amino}acetamide (500g) in dichloromethane, stir the reaction, and add to the reaction Add 30% liquid caustic soda dropwise to the liquid, filter and separate the liquids, and concentrate the lower layer filtrate to dryness to obtain compound 2.
[0030] (2) Add methanol to compound 2, stir to dissolve, add L-tartaric acid (250g) to the solution, heat up and stir until the material is completely dissolved, cool down to crystallize, filter, add the resulting solid to methanol, and heat up to 40-60°C Stir to make a slurry, cool down to -10-10°C to cool and crystallize, and filter to obtain compound 3.
[0031] (3) Add drinking water and dichloromethane to the obtained solid, stir to dissolve, add NaHCO3 aqueous solution to the solution, adjust the pH of the reaction solution to neutral, and add cyclohexylformyl chloride (50g). Stir the reaction, separate the liquid, collect the lowe...
Embodiment 2
[0049] (1) To a toluene solution of N-(2-phenyl)ethyl-2-{(acetal acetal)amino}acetamide (500g), add methanesulfonic acid (2000g) dropwise, stir the reaction, and add to the reaction Add 30% liquid caustic soda dropwise to the solution to neutrality, filter, separate the liquids, and concentrate the filtrate to dryness to obtain Compound 2.
[0050] (2) Add ethanol to compound 2, stir to dissolve, add L-tartaric acid (500g) to the solution, heat up and stir until the material is completely dissolved, cool down to crystallize, filter, add the resulting solid to ethanol, and heat up to 40-60°C Stir to make a slurry, cool down to -10-10°C to cool and crystallize, and filter to obtain compound 3.
[0051] (3) Add drinking water and toluene to the obtained solid, stir to dissolve, add triethylamine to the solution, adjust the pH of the reaction solution to neutral, and add cyclohexylformyl chloride (500g). Stir the reaction, separate the liquid, collect the organic phase, concentra...
Embodiment 3
[0053] (1) In the dichloroethane solution of N-(2-phenyl)ethyl-2-{(acetal acetal)amino}acetamide (100g), add phosphoric acid (500g) dropwise, stir the reaction, and add Add 30% liquid caustic soda dropwise to the reaction liquid until neutral, filter, separate the liquids, and concentrate the lower layer filtrate to dryness to obtain compound 2.
[0054] (2) Add isopropanol to compound 2, stir to dissolve, add L-tartaric acid (200g) to the solution, heat up and stir until the material is completely dissolved, cool down to crystallize, filter, add the resulting solid to isopropanol, and heat up to Stir and beat at 40-60°C, cool down to -10-10°C, cool and crystallize, filter to obtain compound 3.
[0055] (3) Add drinking water and dichloroethane to the obtained solid, stir to dissolve, add NaHCO3 aqueous solution to the solution, adjust the pH of the reaction solution to neutral, and add cyclohexylformyl chloride (100g). Stir the reaction, separate the liquid, collect the orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com