Preparation method of porphyrin aluminum complex and preparation method of polycarbonate
A technology of porphyrin complexes and ligand compounds, which is applied in the field of polymers and can solve problems such as inability to achieve high chemical structure selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
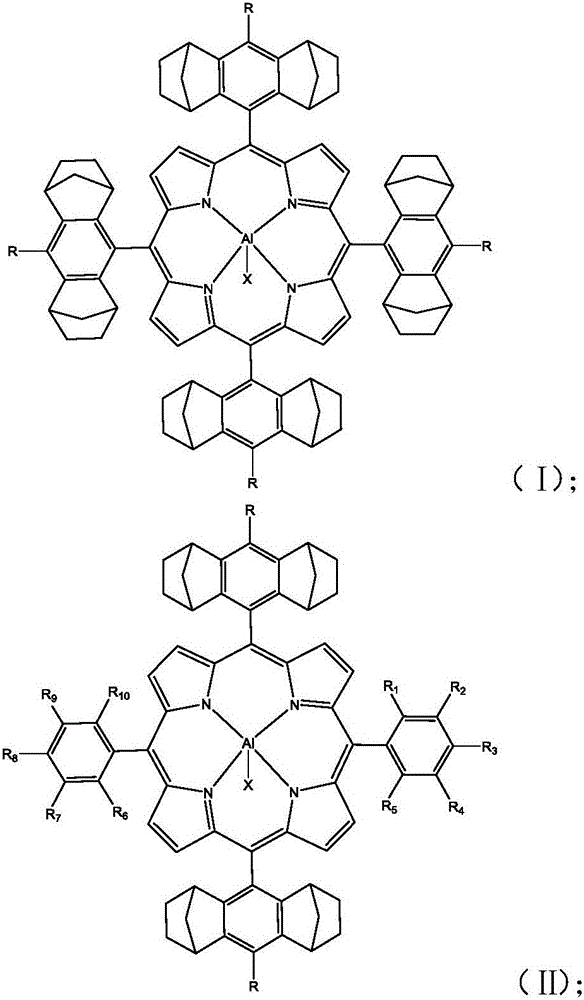
[0068] The preparation method of the aluminum-based porphyrin complex with the structure of formula (I) described in the application comprises the following steps:
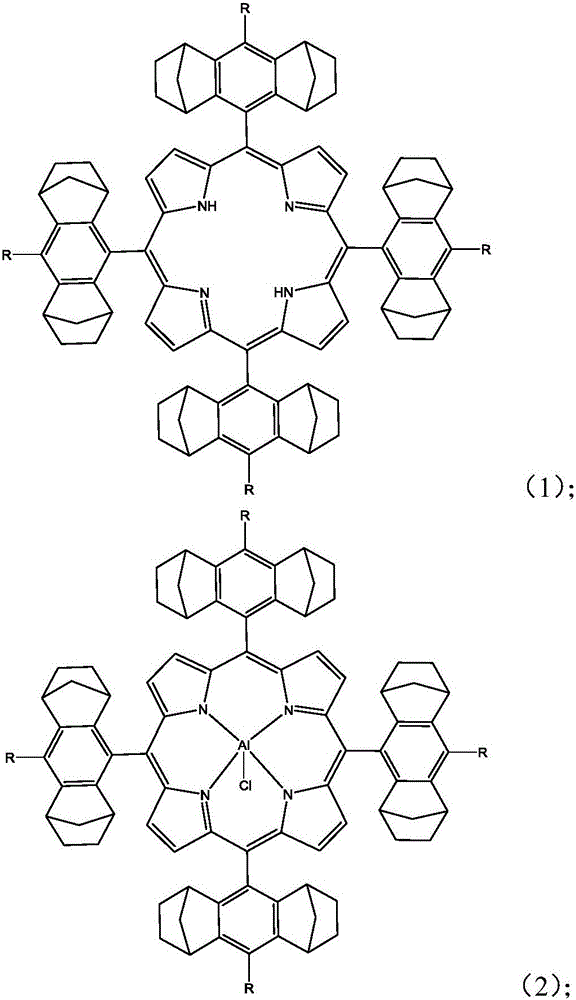
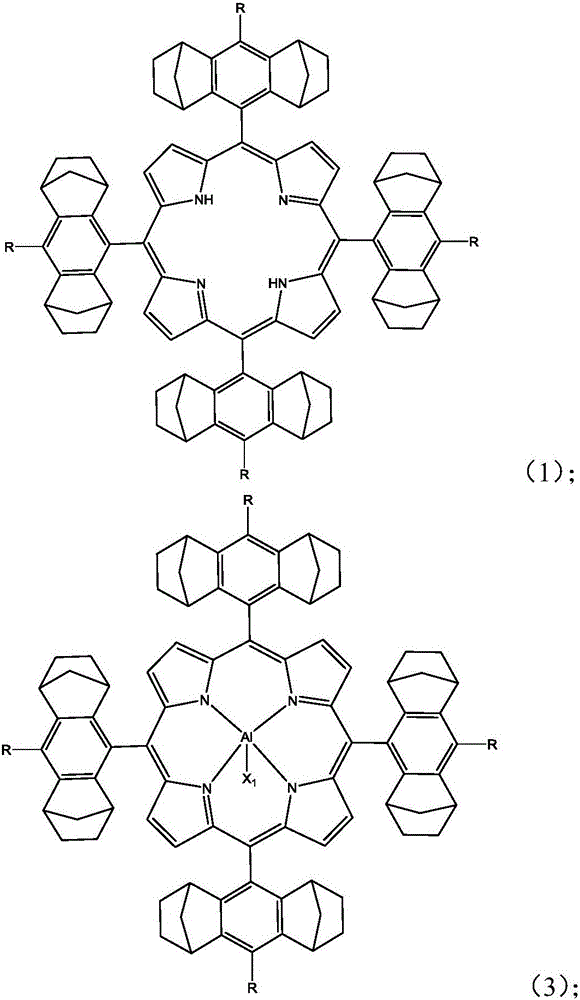
[0069] A), under anhydrous and oxygen-free reaction conditions, compound A and pyrrole are dissolved in a solvent, and the first reaction is carried out under the action of a catalyst to obtain the first compound; the first compound is mixed with 2,3-dichloro- 5,6-dicyano-1,4-p-benzoquinone is subjected to a second reaction to obtain a second compound having a structure shown in formula (1);
[0070] The structural formula of the compound A is
[0071]
[0072] Wherein, R is selected from one of hydrogen, halogen, aliphatic group, substituted aliphatic group, aryl group or substituted aryl group.
[0073] B), the second compound obtained in A) and the metal salt compound are subjected to a third reaction in a solvent to obtain an aluminum-based porphyrin complex having a structure shown in formula (I); X in ...
Embodiment 1
[0133] Compound A (1,2,3,4,5,6,7,8-hydrogen-1:4,5:8-dimethylponthracene-9-carbaldehyde) used in this embodiment has a structure such as formula (17 ), R is hydrogen.
[0134]
[0135] (Step 1-1) Under anhydrous and anaerobic reaction conditions, 1.43g of compound A shown in formula (17) and 0.40g of pyrrole were dissolved in 600ml of dry dichloromethane solvent, followed by compound A and Add 1.2ml trifluoroacetic acid to the reaction solution of pyrrole, and carry out the first reaction at 25°C. After the first reaction time is 1h, add 3.5gDDQ to the obtained first reaction solution, and carry out the second reaction at 25°C , after the second reaction was carried out for 1 h, the obtained second reaction solution was spin-dried to obtain the crude product of the second compound, and then the crude product was purified by silica gel column chromatography to obtain a pure product, and the silica gel column The eluent used in the purification process was n-hexane and dichlo...
Embodiment 2
[0143] Compound A (1,2,3,4,5,6,7,8-Hydro-1:4,5:8-Dimethylpyronthracene-10-methoxy-9-carbaldehyde) used in this example , the structure is shown in formula (19), R is a methoxyl group.
[0144]
[0145] (Step 1-1) Under anhydrous and oxygen-free reaction conditions, 1.61 g of compound A shown in formula (19) and 0.40 g of pyrrole were dissolved in 600 ml of dry dichloromethane solvent, and then compound A and Add 1.2ml trifluoroacetic acid to the reaction solution of pyrrole, and carry out the first reaction at 25°C. After the first reaction time is 1h, add 3.5gDDQ to the obtained first reaction solution, and carry out the second reaction at 25°C , after the second reaction was carried out for 1 h, the obtained second reaction was vacuum removed to remove the solvent to obtain the crude product of the second compound, and then the crude product was purified by silica gel column chromatography to obtain a pure product, and the silica gel column The eluent used in the purific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com