Method for producing glucaric acid
A technology of glucaric acid and aldohexose is applied in the field of preparing glucaric acid and can solve the problems of low preparation rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0060] According to the ratio shown in Table 1, as starting material, glucose was added to the reactor at a concentration of 0.1 g / cc relative to the aqueous solvent, and then potassium hydroxide was added at a ratio of 1:3 mol relative to glucose. Then, a platinum catalyst supported on activated carbon was added at about 30% by weight relative to glucose. Then, the temperature of the reactor was maintained to 50° C., and reacted for 4 hours while oxygen was fed into the reactor and the pressure was maintained at the level of 1 bar.
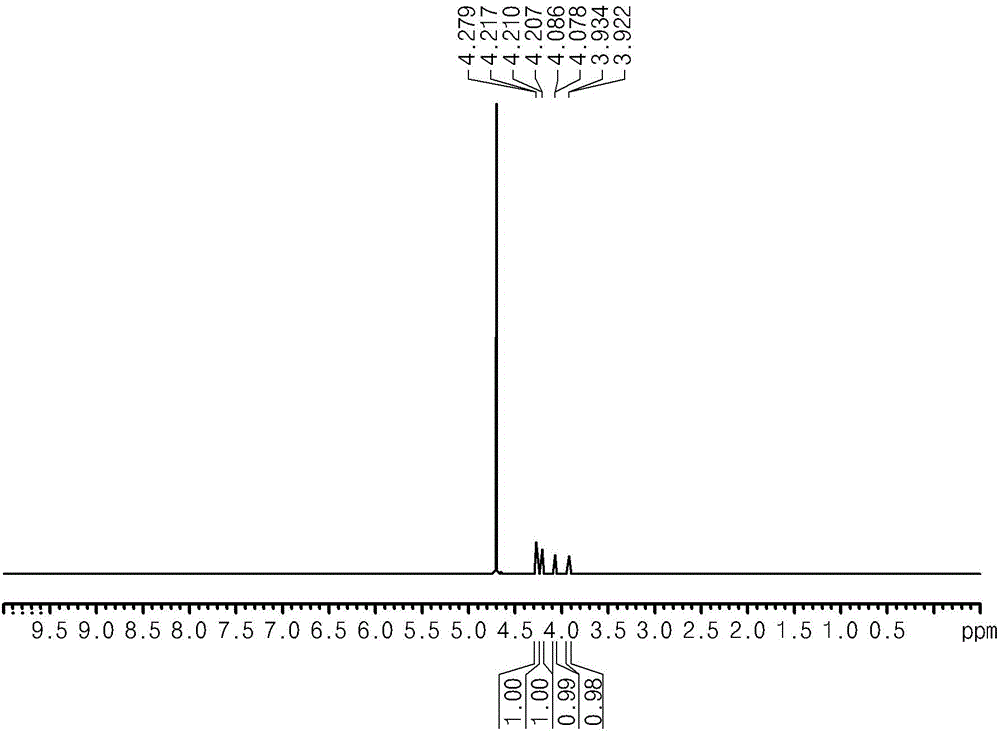
[0061] At the end of the reaction, after separation of water from the reactants, the synthesis of glucaric acid was confirmed by performing NMR analysis (Bruker AVIII400 instrument) and FTIR instrument analysis (Agilent Technologies Cary600) (see figure 1 ). At this point, by dissolving the sample in DMS containing TMS (trimethylsilane) as internal standard 2 NMR spectra were carried out in O ( 1 H at 400MHz).
[0062] - 1 HNMRδ4.14 (d, J =...
Embodiment approach 2
[0068] According to the ratio shown in Table 1, as starting material, glucose was added to the reactor at a concentration of 0.1 g / cc relative to the aqueous solvent, and then potassium hydroxide was added at a ratio of 1:4 mol relative to glucose. Then, a platinum catalyst supported on activated carbon was added at about 50% by weight relative to glucose. Then, the temperature of the reactor was maintained to 50° C., and reacted for 4 hours while oxygen was fed into the reactor and the pressure was maintained at a level of 1.5 bar.
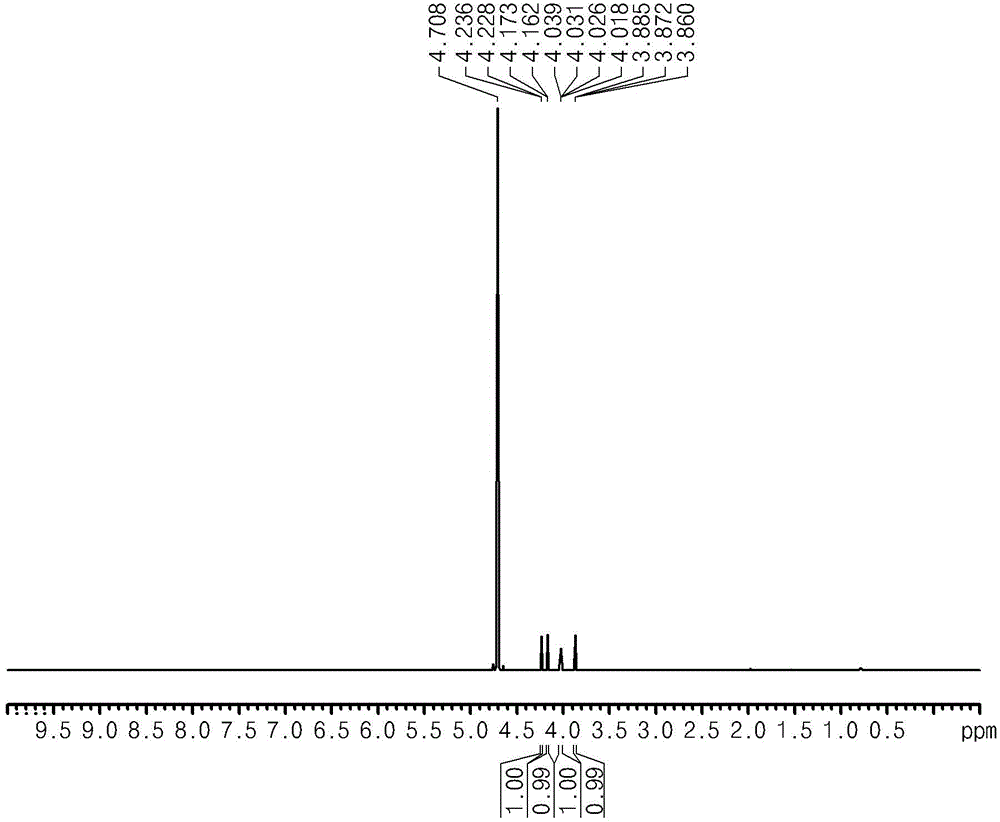
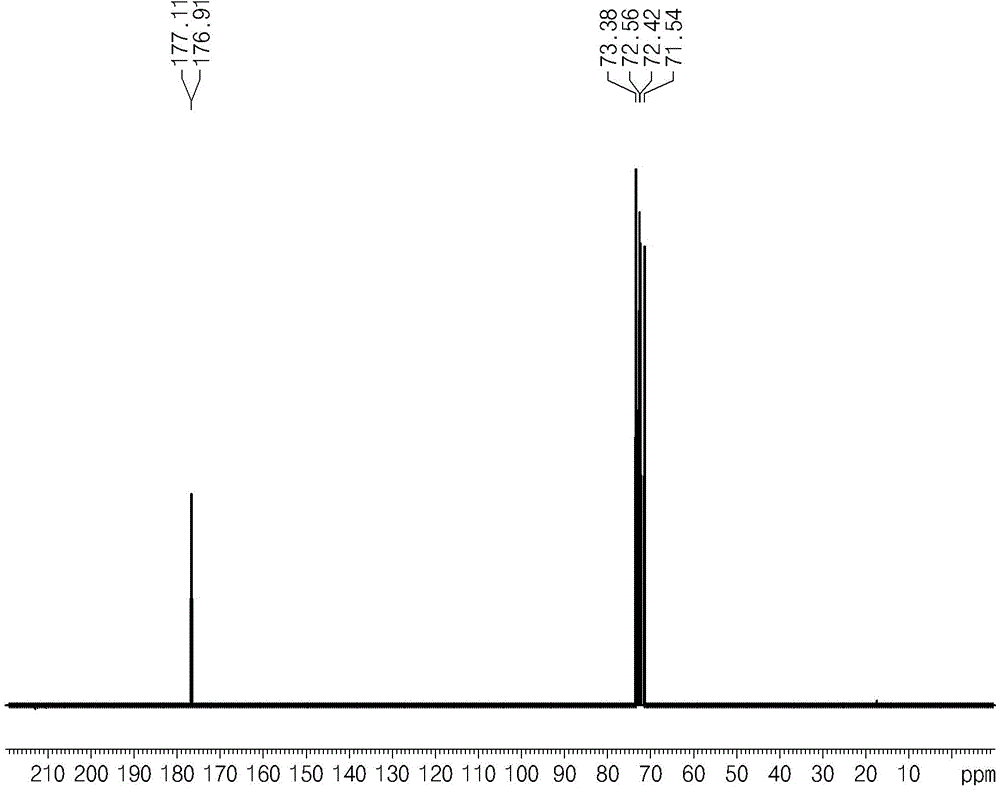
[0069] At the end of the reaction, after separation of water from the reactants, the synthesis of glucaric acid was confirmed by performing NMR analysis (Bruker AVIII400 instrument) and FTIR instrument analysis (Agilent Technologies Cary600) (see figure 2 and image 3 )( 1 H at 400MHz, 13 C at 100MHz).
[0070] - 1 HNMRδ4.14 (d, J = 3.2, 1H),
[0071] 4.09(d,J=4.4,1H),
[0072] 3.96(dd,J=3.2,2.0,1H),
[0073] 3.80 (apparently t,J=5.0). ...
Embodiment approach 3
[0077] According to the ratio shown in Table 1, as starting material, glucose was added to the reactor at a concentration of 0.1 g / cc relative to the aqueous solvent, and then potassium hydroxide was added at a ratio of 1:5 mol relative to glucose. Then, a platinum catalyst supported on activated carbon was added at about 40% by weight relative to glucose. Then, the temperature of the reactor was maintained to 50° C., and reacted for 4 hours while oxygen was fed into the reactor and the pressure was maintained at a level of 2 bar.
[0078] At the end of the reaction, after separation of water from the reactants, the synthesis of glucaric acid was confirmed by performing NMR analysis (Bruker AVIII400 instrument) and FTIR instrument analysis (Agilent Technologies Cary600) (see Figure 4 ).
[0079] - 1 HNMRδ4.14 (d, J = 3.2, 1H),
[0080] 4.09(d,J=4.4,1H),
[0081] 3.96(dd,J=3.2,2.0,1H),
[0082] 3.80 (apparently t,J=5.0).
[0083] -FT-IR (with ATR attachment) 3252, 1742cm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com