Multiple indicator rapid detection method for raw medicinal powder of condensed pill of six drugs with rehmannia
A technology of Liuwei Dihuang Pills and detection methods, which is applied in the field of near-infrared detection, can solve problems such as laborious, difficult production practice, and time-consuming, and achieve the effects of improving production efficiency and economic benefits, stable product quality, and shortening detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
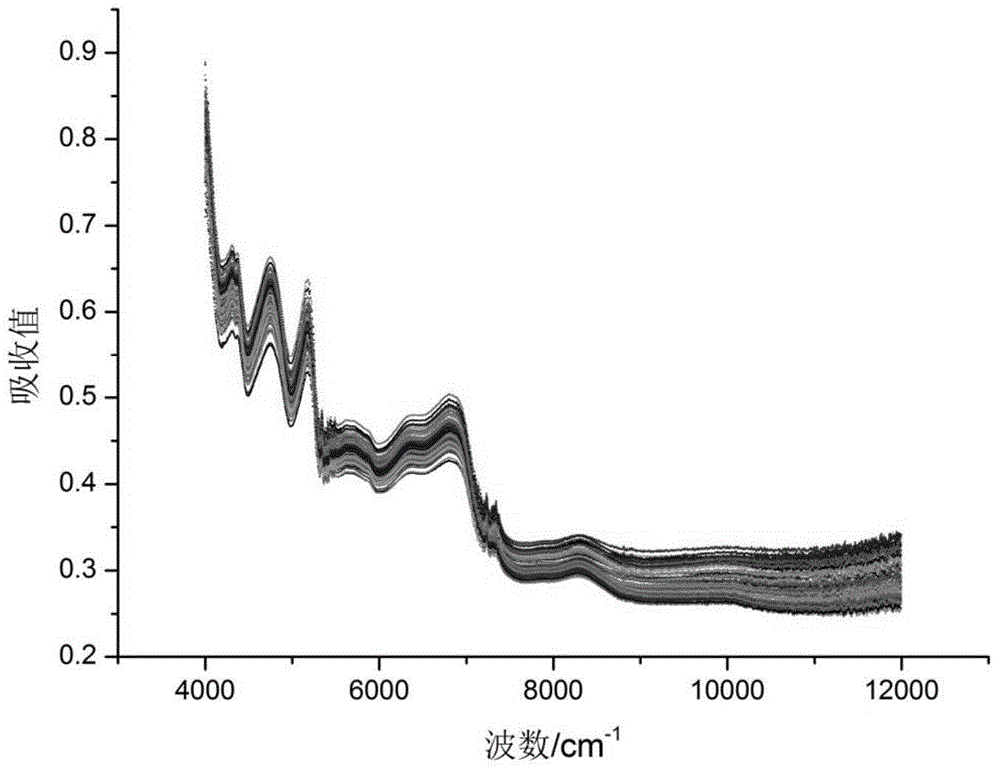
[0037] (1) Collect different production batches of Liuwei Dihuang Pill Concentrated Pill Crude Medicine Powder
[0038]The crude drug powder of concentrated Liuwei Dihuang pills of different production batches was crushed and passed through a 100-mesh sieve to obtain crude drug powder of concentrated Liuwei Dihuang pills with relatively uniform particle size.
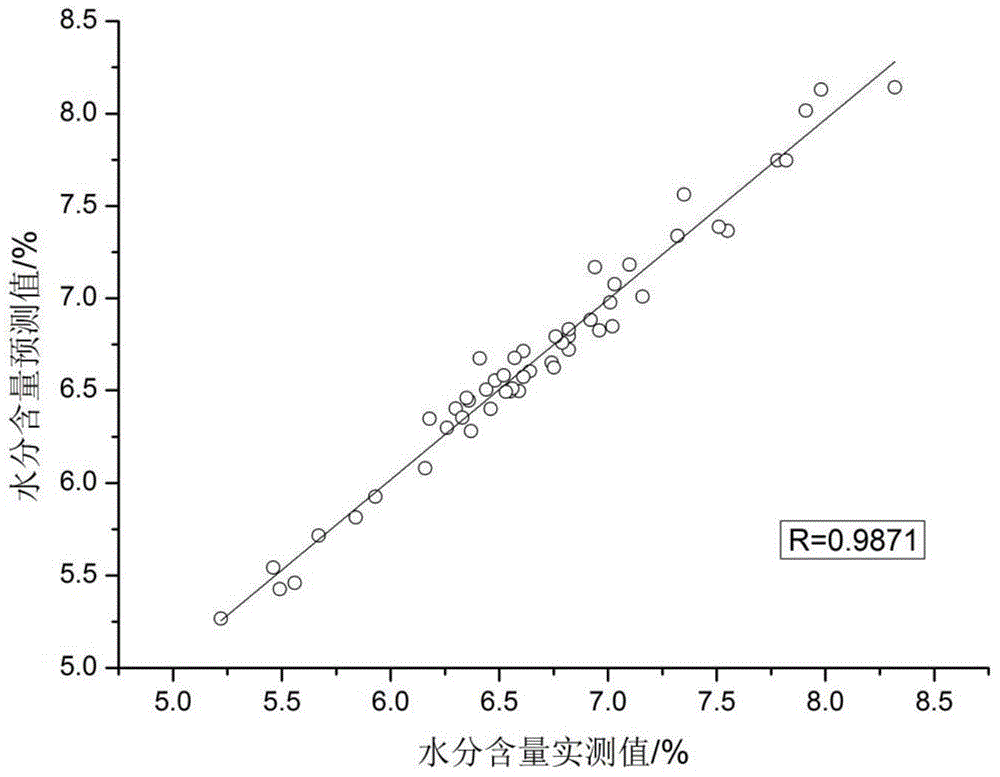
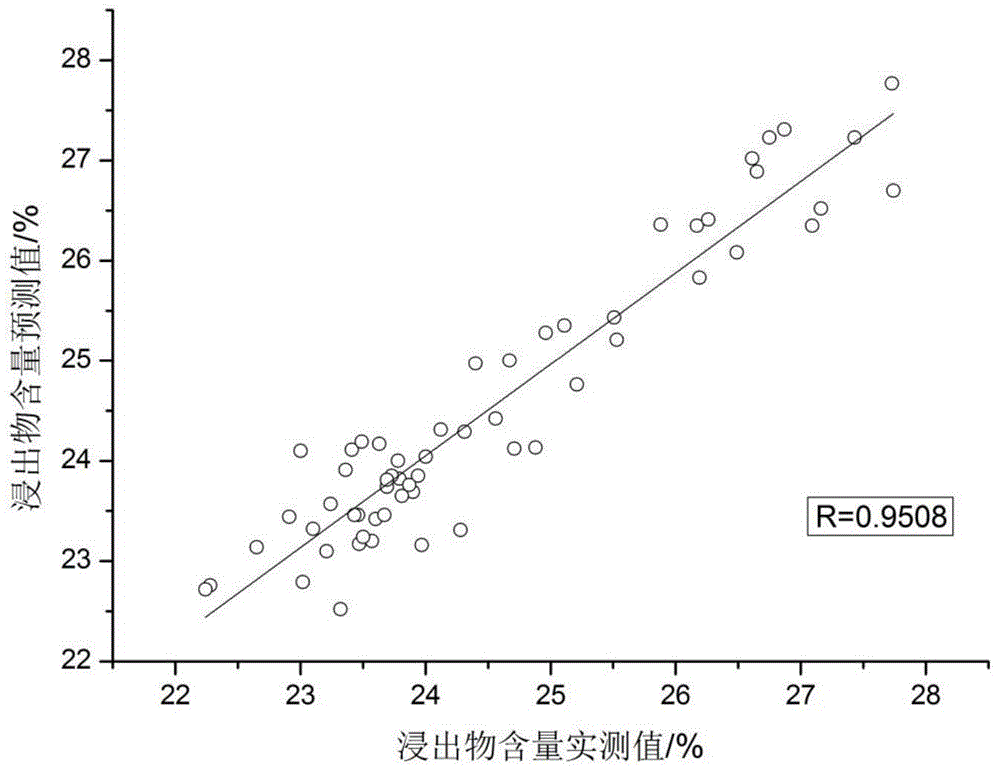
[0039] (2) Determination of key detection indicators of crude drug powder of Liuwei Dihuang Pills Concentrated Pills
[0040] ① Moisture determination method: the moisture content of the crude drug powder of Liuwei Dihuang Pill Concentrated Pills is determined according to the drying and weighing method of the Pharmacopoeia, and the flat bottle (X 0 ), take 2g Liuwei Dihuang Pill Concentrated Pill crude drug powder medical material, accurately weigh (X 1 ), baked in a vacuum oven at 105°C for 5 hours, took it out and cooled it in a desiccator for 30 minutes, weighed, then baked in a vacuum oven for 1 hour, and weighed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com