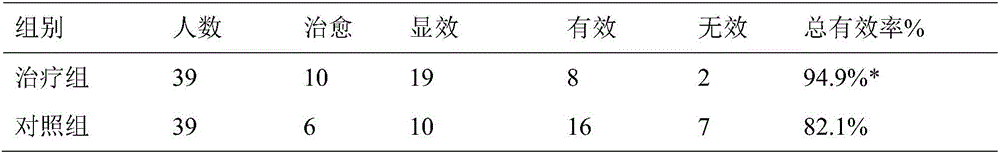
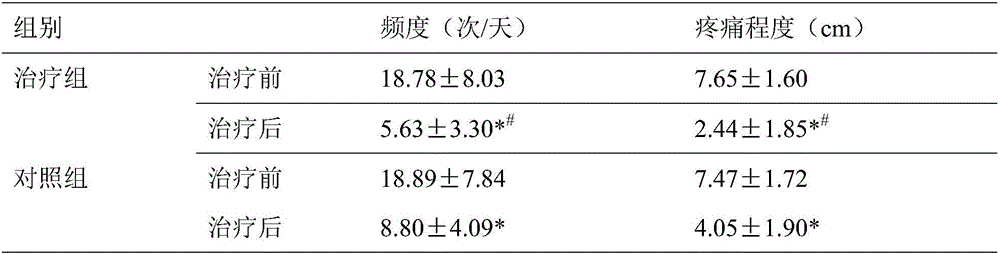
Pharmaceutical composition for treating primary trigeminal neuralgia
A technology for trigeminal neuralgia and composition, applied in the field of traditional Chinese medicine, can solve problems such as damage to cranial nerve or brain tissue, high treatment cost, leukopenia, etc., and achieve the effects of relieving the disease, promoting the smooth flow of qi and blood, and formulating strict prescriptions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 capsule
[0039] Prescription: 12 parts of Rhizoma Chuanxiong, 15 parts of Red Hair Seven, 10 parts of Moutan Cortex, 5 parts of Hexuexiang, 10 parts of Rose, 10 parts of Euonymus, 20 parts of Palm Ginseng, 8 parts of Fangfeng, 9 parts of Tianma, loofah 6 servings.
[0040] Preparation method: take Rhizoma Chuanxiong, Rhododendron chinensis, Cortex Moutan, and Hexuexiang, add 3 to 7 times the weight of medicinal materials, and ethanol with a concentration of 70%, heat and reflux for extraction twice, each time for 1.0 to 1.5 hours, filter, and recover the filtrate Ethanol is concentrated into a thick paste, dried, crushed into a dry paste powder, and set aside;
[0041] Take rose, euonymus, palm ginseng, windproof, gastrodia elata, loofah together with Chuanxiong, Hongmaoqi, moutan cortex, Hexuexiang ethanol to extract the dregs, add 6 to 10 times the weight of the medicinal materials in water, decoct and extract 2 times, filter, concentrate the filtrate ...
Embodiment 2
[0042] Embodiment 2 capsules
[0043] Prescription: 10 parts of Rhizoma Chuanxiong, 12 parts of Hongmaoqi, 10 parts of Moutan Bark, 6 parts of Hexuexiang, 12 parts of Rose, 12 parts of Euonymus, 18 parts of Palm Ginseng, 8 parts of Fangfeng, 9 parts of Gastrodia elata, loofah 6 servings.
[0044] Preparation method: refer to the operation of Example 1.
Embodiment 3
[0045] Embodiment 3 capsules
[0046] Prescription: 10 parts of Rhizoma Chuanxiong, 12 parts of Hongmaoqi, 12 parts of Moutan Cortex, 4 parts of Hexuexiang, 10 parts of Rose, 15 parts of Euonymus, 15 parts of Palm Ginseng, 6 parts of Fangfeng, 8 parts of Gastrodia elata, loofah 6 servings.
[0047] Preparation method: refer to the operation of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com