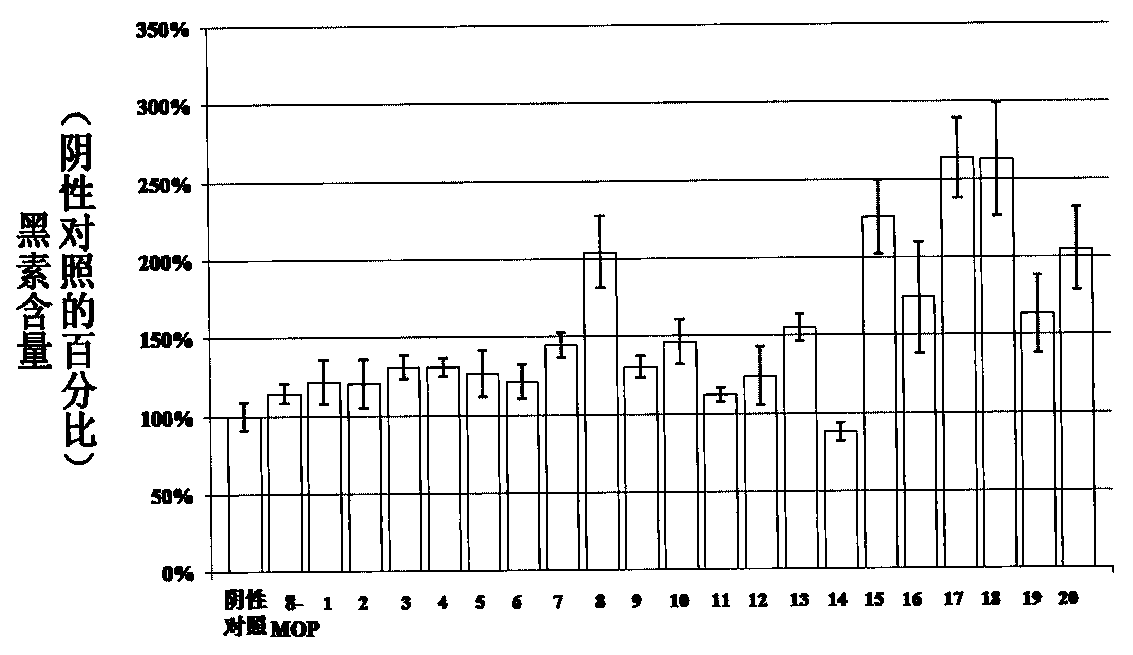
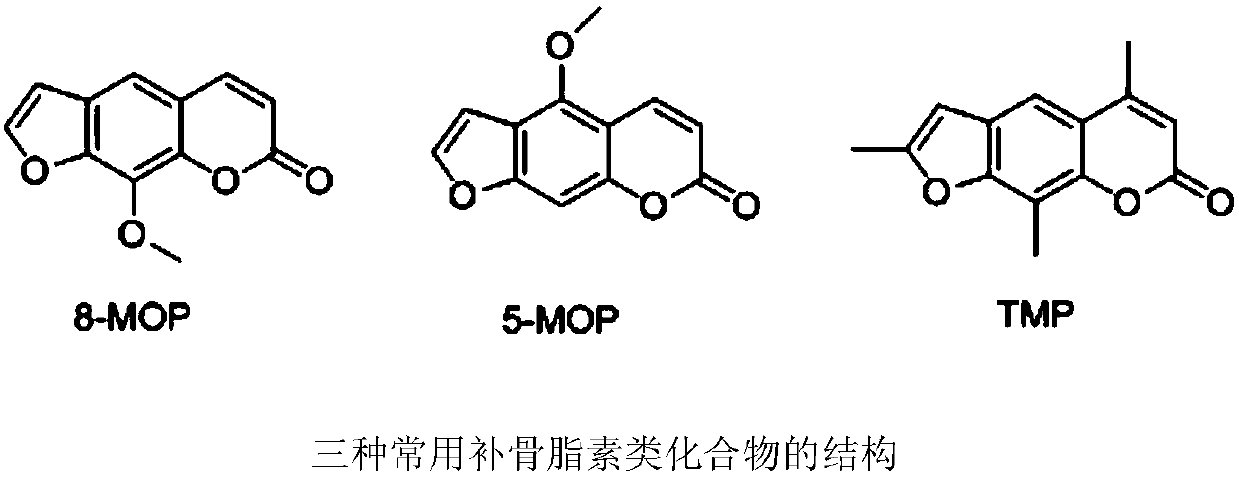
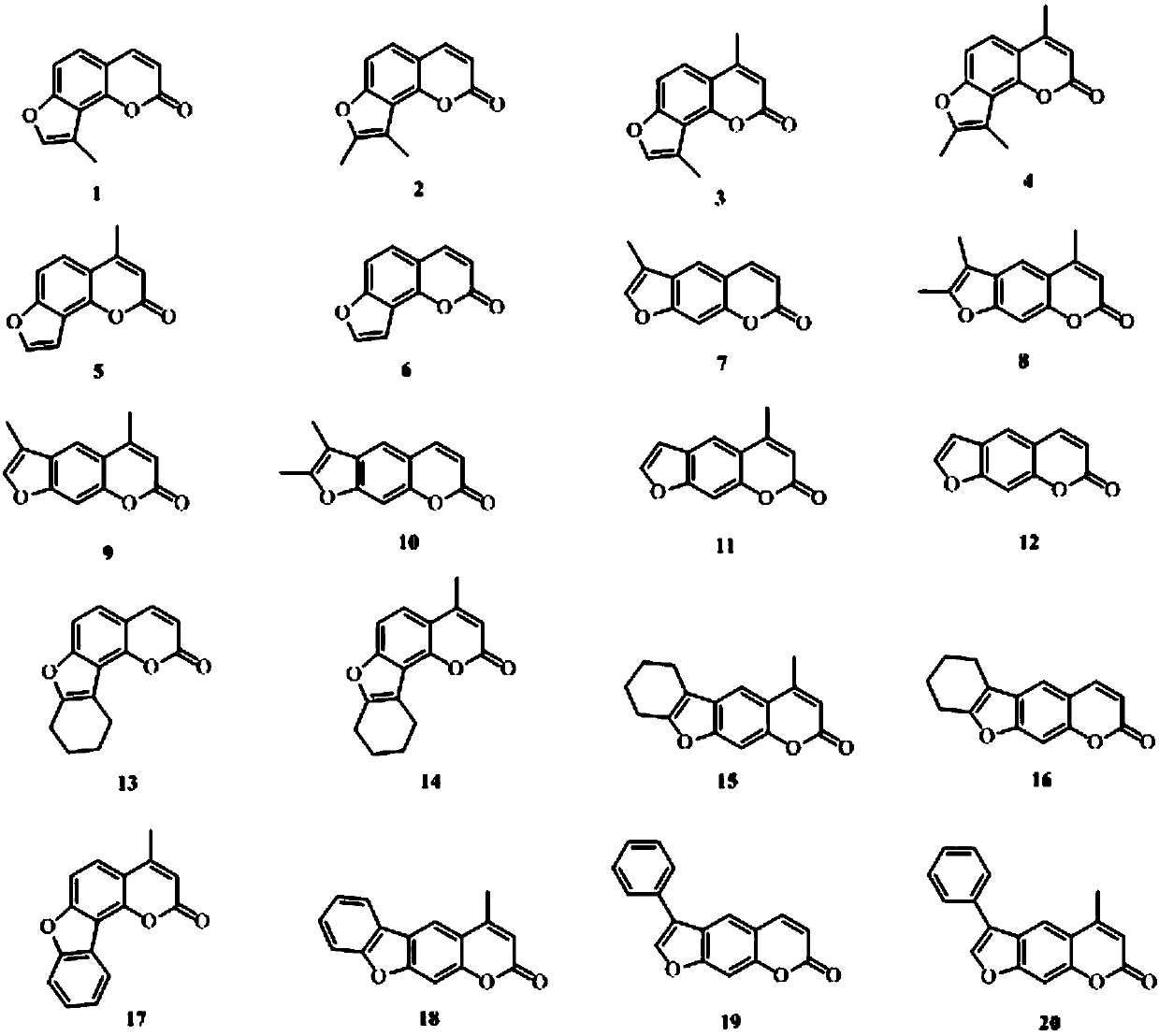
A kind of application of psoralen compound
A technology for psoralen and compound, which is applied in the directions of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, drug combinations, etc. Problems such as unclear process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] 9-methyl-2H-furo[2,3-h]benzopyran-2-one (compound 1) and 6-methyl-2H-furo[3,2-g]benzopyran-2- Preparation of ketone (compound 7):
[0084] At room temperature, dissolve 0.11g (1mmol) of resorcinol in 20mL of dry sulfuric acid, stir until completely dissolved, then add 0.161g (1.2mmol) of malic acid, raise the temperature to 120°C, stir well, and detect the reaction by TLC After completion, cool to room temperature, pour the reaction solution into ice water, extract with ethyl acetate after standing, dry with anhydrous sodium sulfate, and remove the solvent in vacuum to obtain 0.140 g of 7-hydroxycoumarin;
[0085] 0.81g (5mmol) 7-hydroxycoumarin and 5g potassium carbonate (36mmol) were dissolved in 50mL acetone, and 0.48mL (6mmo) 2-chloroacetone was added, fully stirred, after TLC detection reaction was complete, cooled to room temperature, The reaction solution was filtered and concentrated, and the residue was eluted with a gradient gradient of petroleum ether:ethyl ...
Embodiment 2
[0094] 8,9-Dimethyl-2H-furo[2,3-h]benzopyran-2-one (compound 2) and 6,7-dimethyl-2H-furo[3,2-g]benzene Preparation of pyran-2-one (compound 10):
[0095] At room temperature, dissolve 0.11g (1mmol) of resorcinol in 20mL of dry sulfuric acid, stir until completely dissolved, then add 0.161g (1.2mmol) of malic acid, raise the temperature to 120°C, stir well, and detect the reaction by TLC After completion, cool to room temperature, pour the reaction solution into ice water, extract with ethyl acetate after standing, dry with anhydrous sodium sulfate, and remove the solvent in vacuum to obtain 0.140 g of 7-hydroxycoumarin;
[0096] Dissolve 0.81g (5mmol) of 7-hydroxycoumarin and 5g of potassium carbonate (36mmol) in 50mL of acetone, and add 0.60mL (6mmo) of 2-chlorobutanone, stir well, and after TLC detects that the reaction is complete, cool to room temperature , the reaction solution was filtered and concentrated, and the residue was eluted with petroleum ether at a volume rat...
Embodiment 3
[0105] 8,9,10,11-tetrahydro-2H-benzofuro[2,3-h]benzopyran-2-one (compound 13) and 6,7,8,9-tetrahydro-2H-benzene Preparation of furo[3,2-g]benzopyran-2-one (compound 16):
[0106] At room temperature, dissolve 0.11g (1mmol) of resorcinol in 20mL of dry sulfuric acid, stir until completely dissolved, then add 0.161g (1.2mmol) of malic acid, raise the temperature to 120°C, stir well, and detect the reaction by TLC After completion, cool to room temperature, pour the reaction solution into ice water, extract with ethyl acetate after standing, dry with anhydrous sodium sulfate, and remove the solvent in vacuum to obtain 0.140 g of 7-hydroxycoumarin;
[0107] 0.81g (5mmol) of 7-hydroxycoumarin and 5g of potassium carbonate (36mmol) were dissolved in 50mL of acetone, and 0.70mL (6mmo) of 2-bromocyclohexanone was added, fully stirred, and after TLC detected that the reaction was complete, it was cooled to At room temperature, the reaction liquid was filtered and concentrated, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com