Human pancreatic cancer nude mouse model construction method and use thereof
A construction method and pancreatic cancer technology, applied in the field of construction of human pancreatic cancer nude mouse model, can solve the problems of high tumor formation rate, low cell tumor formation rate, and long tumor formation incubation period, and achieve high tumor formation rate, tumor formation fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the determination of puromycin optimal screening concentration
[0034] (1) Materials: puromycin, human pancreatic cancer PANC-1 cells (purchased from ATCC, USA).
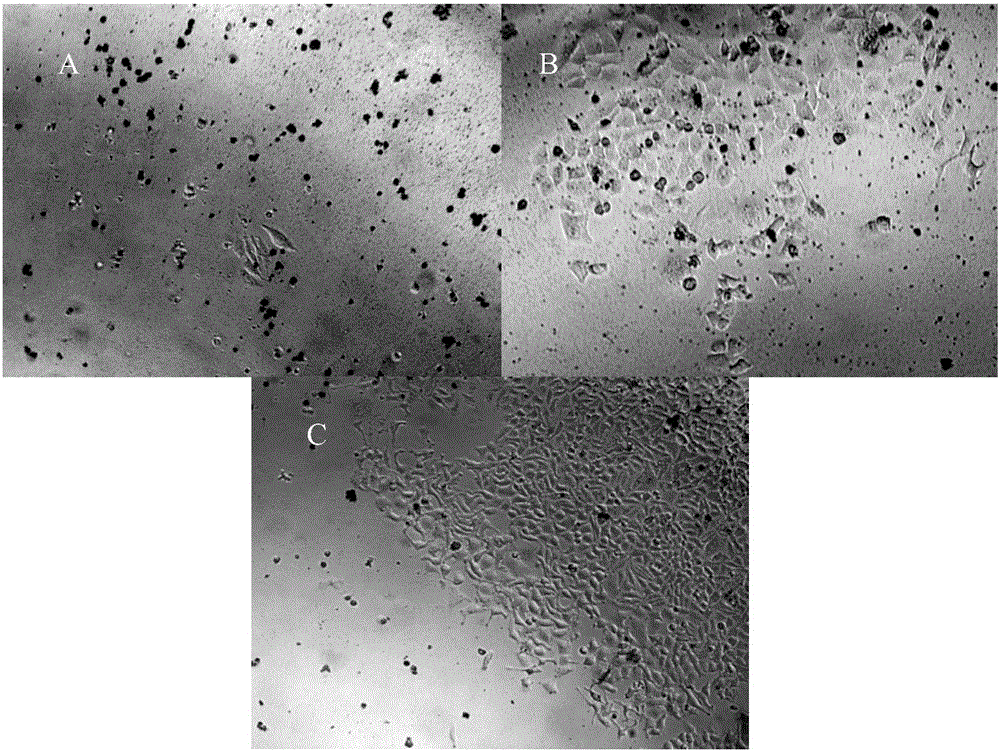
[0035] (2) Experimental method: Digest the PANC-1 cells in the logarithmic growth phase with 0.25% trypsin, inoculate them in a 6-well plate at a cell density of 50%, add DMEM containing 10% fetal bovine serum to culture in high glucose Incubate overnight at 37°C in a 5% CO2 incubator. On the second day, different concentrations of puromycin (1 μg / ml, 2.5 μg / ml, 5 μg / ml, 7.5 μg / ml, 10 μg / ml) were added, and new media containing different concentrations of puromycin were replaced every 2 days. The minimum concentration at which all cells died within 4 days was selected as the screening concentration.
[0036] (3) Experimental results: On the second day of culture, a large number of cells died in the wells with a dose of 5 μg / ml or more of puromycin. After 3 days of culture, different degrees ...
Embodiment 2
[0037]Example 2: Construction of PANC-1 cells stably expressing luciferase
[0038] (1) Materials: plasmid vector GV258 with luciferase gene, puromycin, Nucleofector TM Solution SE Kit.
[0039] (2) Experimental method
[0040] 1.1 Cell transfection
[0041] Take the 4th passage PANC-1 cells after recovery, digest the cells with 0.25% trypsin+0.02% EDTA, collect the cells by centrifugation at 1000r / min for 5min, take 5×10 6 cells, flick the bottom of the tube to mix the cells to Resuspend the cells in SE Solution, add 8 μg of the GV258 plasmid vector expressing luciferase, gently add the cell suspension to the electroporation cup, 4D-Nucleofector TM System nucleofection system for cell transfection with DN-100 program. Transfer the cells in the electroporation cup to a 6-well cell culture plate with 37°C preheated medium, 37°C, 5% CO 2 Cultivate in the incubator for 48h.
[0042] 1.2 Screening of PANC-1 cells stably expressing luciferase
[0043] Collect the PANC-1 ...
Embodiment 3
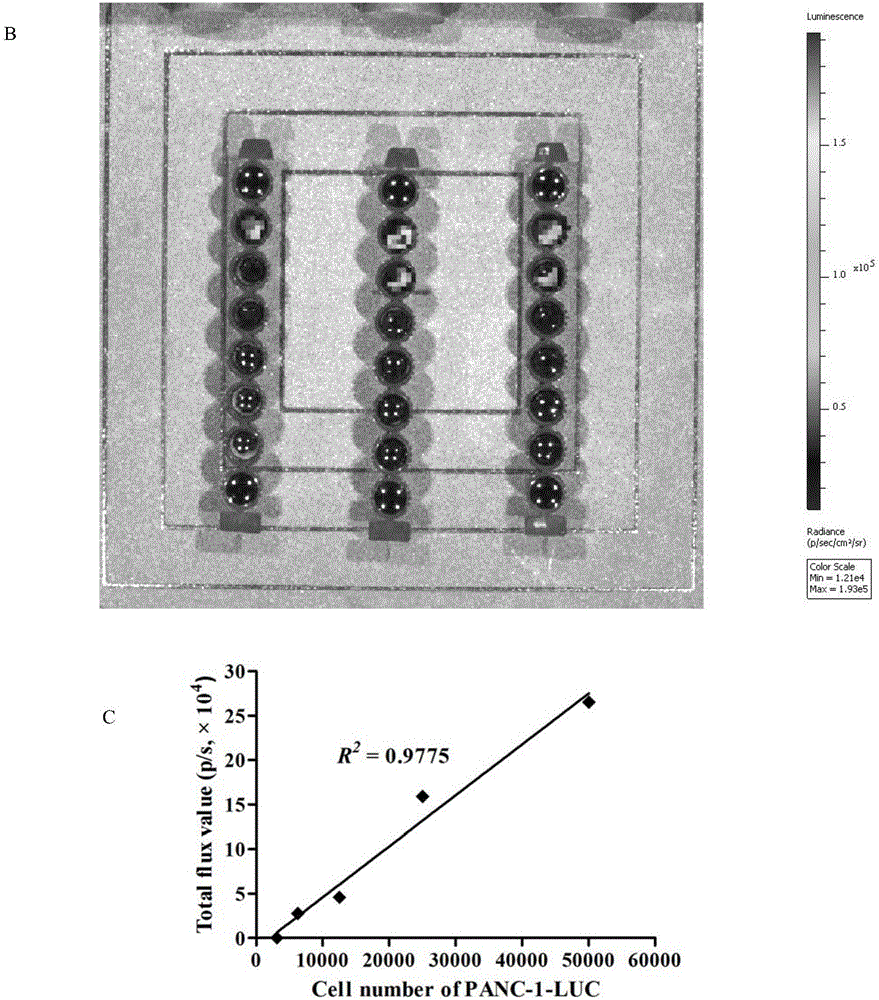
[0046] Example 3: In vivo imaging system detects the expression of luciferase in PANC-1-LUC cells
[0047] (1) Materials: PANC-1-LUC cells, D-fluorescein potassium salt, isoflurane.
[0048] Experimental animals: SPF grade BALB / c-nu / nu nude mice, male, 4-5 weeks old.
[0049] (2) Experimental method
[0050] 1.1 In vitro bioluminescence detection
[0051] Digest and count the PANC-1-LUC cells in the logarithmic growth phase, and adjust the cell concentration to 5×10 5 cells / ml, 100 μl per well was inoculated in a black 96-well plate, and the cells were sequentially diluted one by one. Three parallel wells were set up for each order of magnitude of cells, and 100 μl of D-luciferin (150 μg / ml) was added to each well. detected in the imaging system.
[0052] 1.2 In vivo bioluminescence detection
[0053] Digest and count the PANC-1-LUC cells in the logarithmic growth phase, and adjust the cell density to 5×10 7 cells / ml, and then diluted to 2.5×10 7 pcs / ml, 1×10 7 pcs / ml,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com