Application of indolyl diketopiperazine compound in preparation of antifungal medicines
A technology of antifungal drugs and compounds, applied in the field of medicine and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
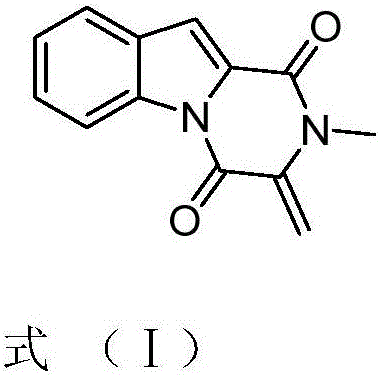
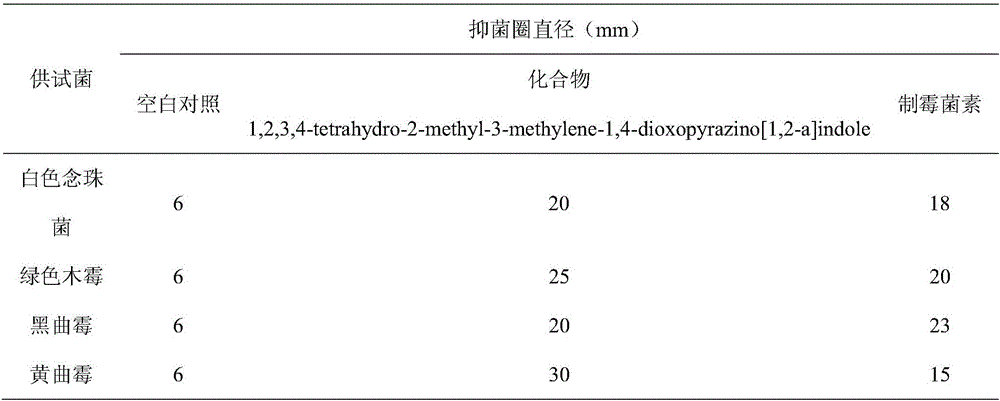
[0016] The effect of compound 1,2,3,4-tetrahydro-2-methyl-3-methylene-1,4-dioxopyrazino[1,2-a]indole on Candida albicans, Trichoderma viride, Aspergillus niger or Aspergillus antibacterial activity.
[0017] The compound 1,2,3,4-tetrahydro-2-methyl-3-methylene-1,4-dioxopyrazino[1,2-a]indole was dissolved in DMSO and diluted to a concentration of 2 mg / mL.
[0018] Take Candida albicans in the logarithmic growth phase and prepare them with physiological saline to a concentration of 10 6 CFU / mL bacterial solution; other fungi prepared to spore number of 10 6 spore suspension per mL, draw 1 mL of the prepared bacterial liquid or spore suspension into a petri dish, pour it into the PDA medium that has been cooled to a suitable temperature, mix well, and use sterile tweezers to pick up 1.5 mm thick, A filter paper piece with a diameter of 6 mm was placed on the bacteria-containing plate, and 5 μL of the extract was added dropwise on the filter paper as a sample group, DMSO was use...
Embodiment 2
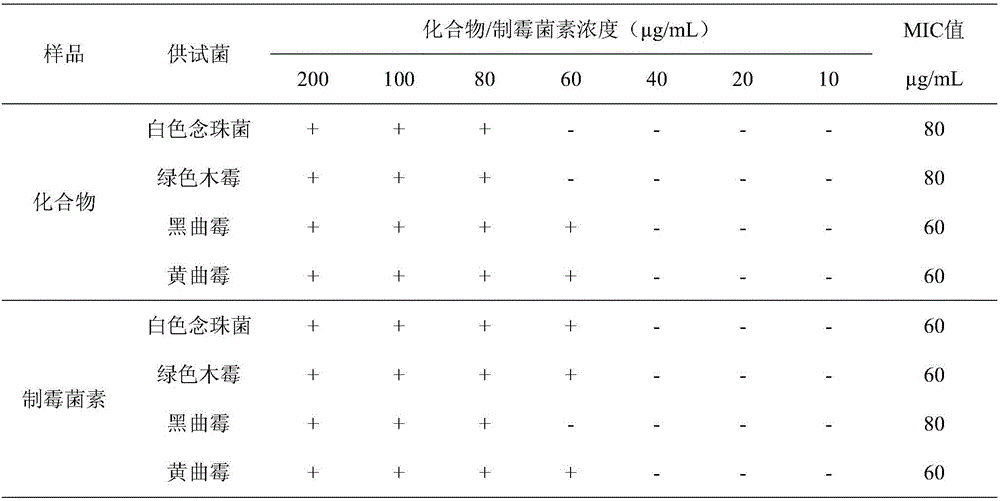
[0023] The effect of compound 1,2,3,4-tetrahydro-2-methyl-3-methylene-1,4-dioxopyrazino[1,2-a]indole on Candida albicans, Trichoderma viride, Aspergillus niger, The minimum inhibitory concentration of Aspergillus.
[0024] Compound 1,2,3,4-tetrahydro-2-methyl-3-methylene-1,4-dioxopyrazino[1,2-a]indole or nystatin were diluted to 200, 100, 80, 60, 40, 20, 10 μg / mL. The absorption concentration is 10 6 CFU / mL of Candida albicans solution or 10 6 Put 1mL of other fungal spore suspensions / mL into the plate, pour into the PDA medium that has been cooled to an appropriate temperature, and mix well. Use sterile tweezers to pick up a filter paper sheet with a thickness of 1.5 mm and a diameter of 6 mm and place it on a plate containing bacteria, and at the same time add the above-mentioned concentration of compound 1,2,3,4-tetrahydro-2-methyl-3 to the filter paper sheet respectively -methylene-1,4-dioxopyrazino[1,2-a]indole or nystatin 5 μL, cultured in a 28°C incubator for 72 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com