Thrombolytic drug based on cobra venom PIIII type metalloproteinase and application of thrombolytic drug
A technology of metalloprotease and cobra venom, applied in the direction of peptide/protein components, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of unusable preparation and achieve a significant effect of thrombolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
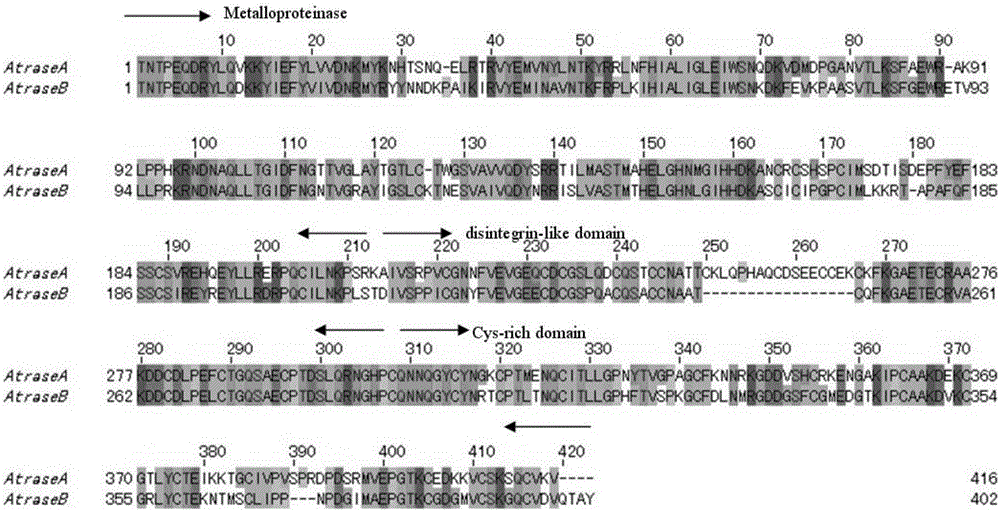
[0033] The target protein is obtained by separating and purifying from the snake venom of Chinese cobra (Zhoushan species cobra) (classification name: Naja atra). The target protein is a single-chain glycoprotein, and its structural analysis shows that it has three structural domains: a metalloprotease domain, a disintegrin-like domain, and a cysteine-rich domain, belonging to the PIII type snake venom metalloprotease ( figure 1 ).
[0034] Table 1 The physical and chemical indicators of the target protein deduced from the primary structure
[0035]
[0036] As shown in Table 1: the target protein has the activity of hydrolyzing the alpha chain of fibrinogen, which can be completely inhibited by metal chelating agents EDTA, EGTA, 1,10-phenanthroline and reducing agent DTT. The actual molecular weights of the target proteins Atrase A and Atrase B are about 49.3kDa and 49.4kDa respectively, and their sequences are: AtraseA: SEQIDNo.1; AtraseB: SEQIDNo.2; however, the apparen...
Embodiment 2
[0052] 1. The role of cobra venom type PIII metalloproteinases in inducing endothelial cell fibrinolysis (taking Atrase A as an example)
[0053] 1.1 Materials and reagents
[0054] Human microvascular endothelial cells (HMEC); RPMI-1640 medium was purchased from Gibco; fetal bovine serum (FBS) was a product of Tianjin Haoyang Biological Products Technology Co., Ltd.; t-PA (tissue plasminogen activator ), PAI-1 (plasminogen activator inhibitor) content detection ELISA kit and activity assay kit were purchased from Assaypro Company of the United States; normal human serum (normal human serum, NHS) was prepared from the blood donation of several healthy volunteers, and frozen Stored at -80°C, tested for normal complement activity, ready for use; inactivated normal human serum (INHS) was obtained by incubating NHS at 56°C for 30 minutes; the separation and purification of Atrase A was the same as before; the rest of the reagents were in compliance with Analytical purity required...
Embodiment 3
[0077] 1. The pharmacodynamic effect of cobra venom type PIII metalloprotease on thrombolysis in vivo (taking Atrase A as an example)
[0078] 1.1 Experimental materials
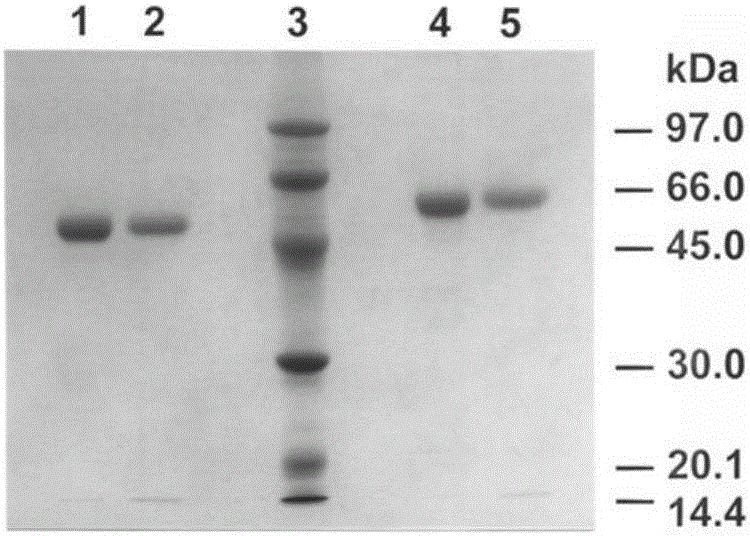
[0079] The preparation of the thrombolytic drug Atrase A was the same as before, and the purity of the SDS-PAGE test was a single electrophoresis band, and the purified samples were subpackaged and stored at -80°C; aspirin was purchased from Sigma, USA; urokinase (UK) was Livzon The product of Livzon Pharmaceutical Factory of the Group; the recombinant human tissue plasminogen activator alteplase (rt-PA) is a product of Boehringer Ingelheim, Germany; other reagents are all reagents or drugs that meet the experimental requirements.
[0080] SPF-grade male SD rats were purchased from Chongqing Tengxin Experimental Animal Co., Ltd., weighing 210-240 g, with certificate number SCXK (Yu) 2007-0005. Animal experiments and animal welfare complied with relevant animal experiment management regulations. The experim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com