Tofacitinib citrate
A technology of cloth citrate and cloth citric acid, which is applied in the field of medicine, can solve the problems of poor viscosity and fluidity, and is not suitable for direct compression, and achieves the effects of good dissolution and dissolution performance, good dissolution rate, and good fluidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0135] Embodiments of the present invention first disclose tofacitinib citrate having a median particle size of less than 40 μm. Although various embodiments have been disclosed in the present invention, various adjustments and modifications can be made by those skilled in the art within the scope of the present invention based on common knowledge in the art. The modification includes equivalent replacement of any aspect of the invention with a solution that is basically the same or included in the present invention that is obvious to those skilled in the art in order to achieve substantially the same effect. Numerical ranges are inclusive of the numerical values defining the range. Furthermore, numerical ranges are provided such that in addition to reciting individually specific numerical values within the range, also numerical ranges outside the range are also recited.
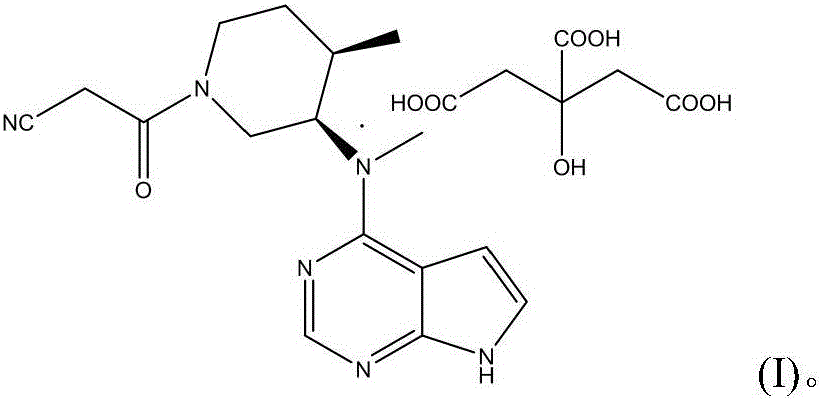
[0136] N-((3R4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine as dis...
Embodiment A
[0137] The preparation of embodiment A Tofacitinib citrate
[0138] Into a round bottom flask equipped with hydrogen was added 10% palladium hydroxide on carbon (0.56 g), methanol (20 ml), acetic acid (0.5 g) and N-methyl-N-{(3R,4R)-1-benzyl Base-4-methylpiperidin-3-yl}-7H-pyrrole[2,3-d]pyrimidin-4-amine (2.8g), was first replaced by nitrogen, and then hydrogen (1atm). The resulting mixture was reacted at 50°C for about 6 hours. After the reaction was complete, the solid was removed by filtration, the filtrate was concentrated, and ethanol (20ml) was added to the obtained concentrate. Monocarb-7-ene (3.8g), ethyl cyanoacetate (3.7g), and the mixture was stirred at room temperature for about 6 hours. Add citric acid (3.5g) and water (18ml) directly without treatment, stir at room temperature, and a solid precipitates, which is filtered to obtain tofacitinib citrate. The particle size distribution measurement results are as follows: D50 is 56.973 μm, D10 is 17.348 μm, D90 is 2...
Embodiment 1
[0139] Example 1: Preparation of Tofacitinib Citrate Form A
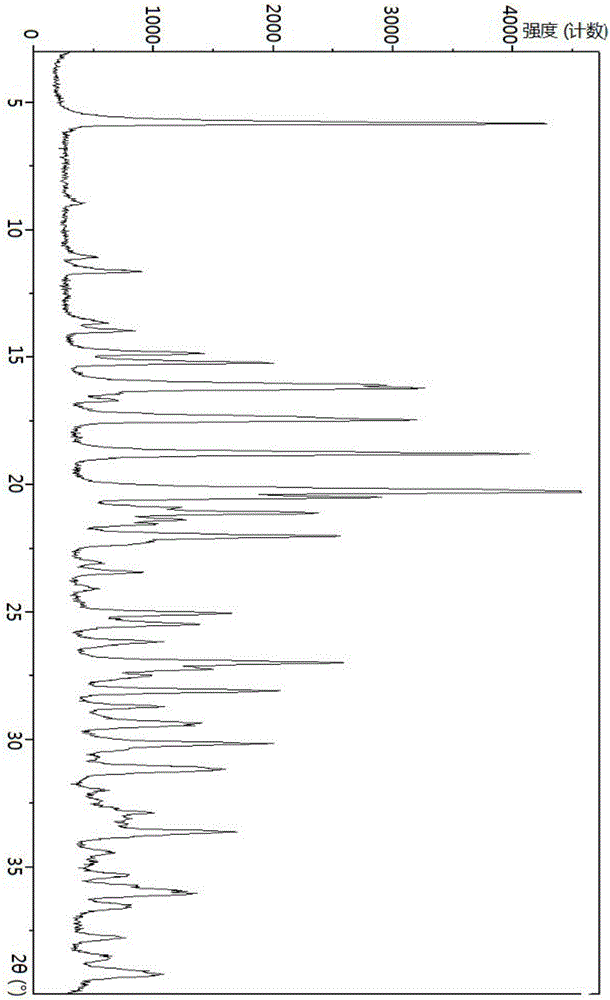
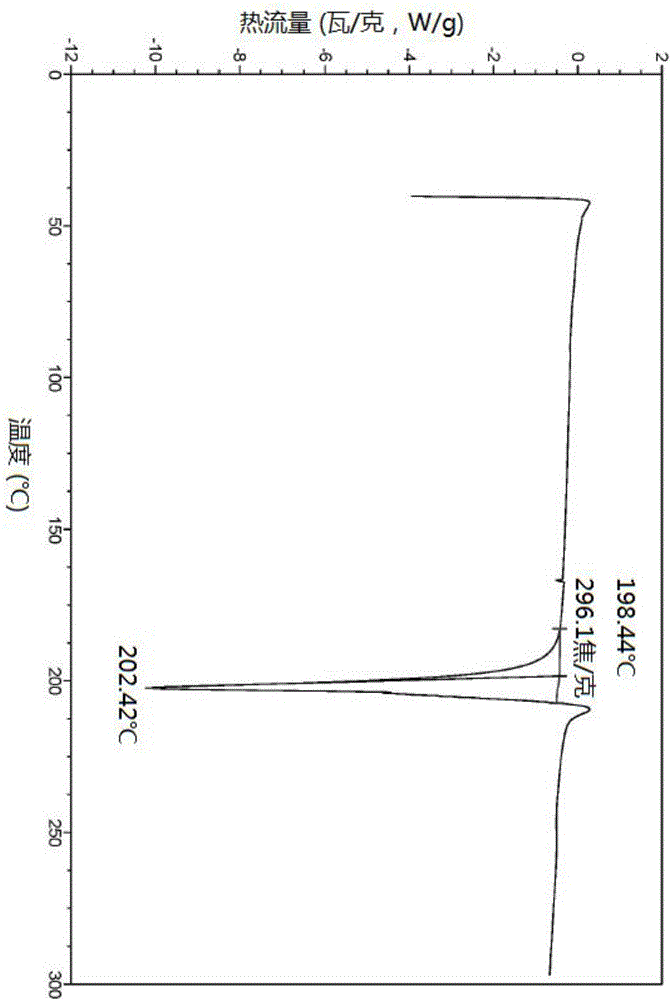
[0140] Tofacitinib citrate (10g, prepared according to the method of Example A), ethanol (100ml), water (60ml), was added to a round bottom flask to form a mixed solution, stirred at a speed of 300rpm and heated to reflux to form a solution, The solution was stirred at reflux temperature for 1 hour and then cooled to 40°C. After being kept at 40°C for 1 hour, the mixture was cooled to 10°C and maintained at this temperature for 2 hours, filtered, and vacuum-dried at 45°C for 10 hours to obtain tofacitinib citrate Crystal 9.52g. The X-ray powder diffraction pattern of gained tofacitinib citrate crystal is shown in figure 1 .
[0141] The particle size distribution results of the obtained tofacitinib citrate crystals are: D50 is 5.816 μm, D10 is 1.837 μm, D90 is 16.782 μm, detection instrument: Malvern Mastersizer 2000 particle size analyzer, dispersion pressure is 3.0Bar, sample injection The rate is 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com