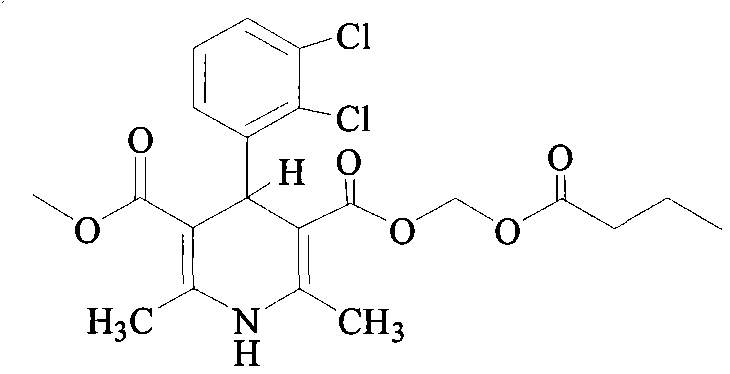
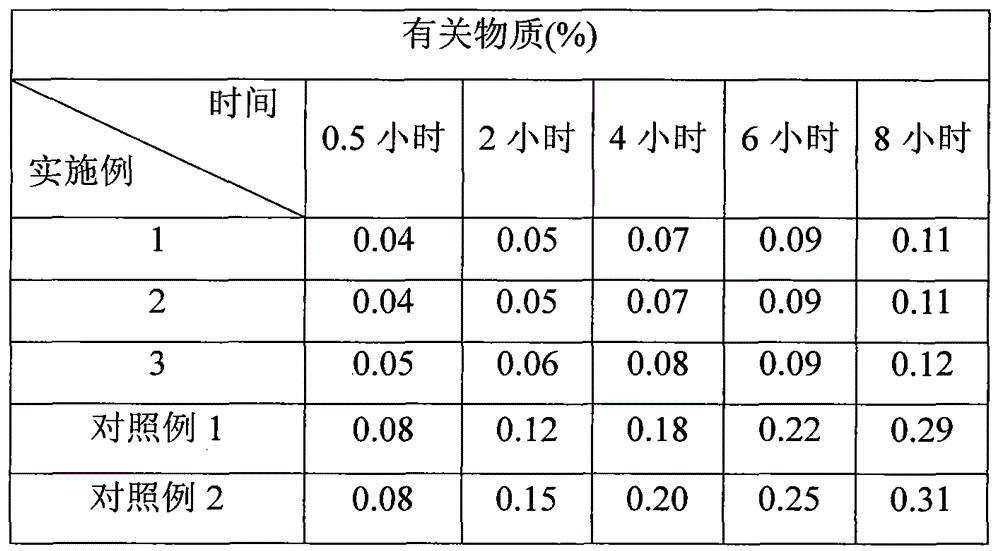
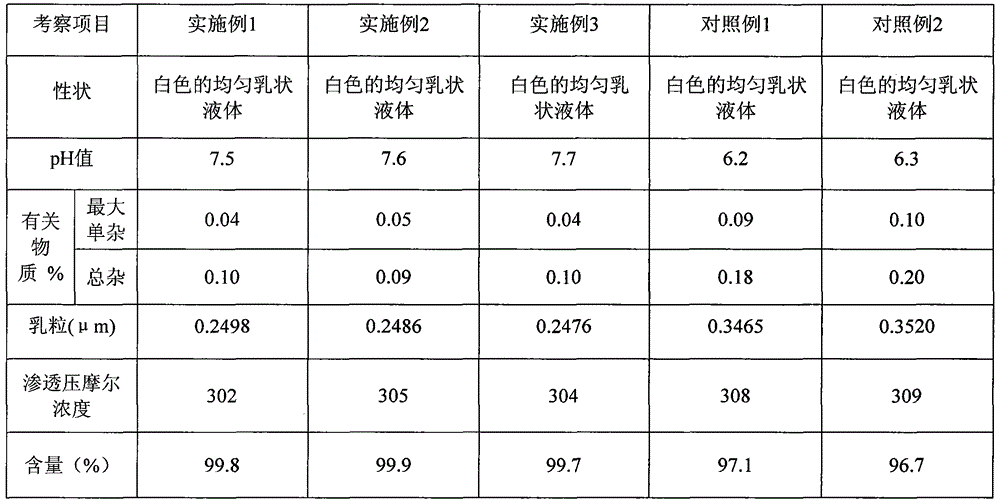
Preparation method of clevidipine butyrate injection fat emulsion
A technology of clevidipine butyrate and fat for injection, which is applied in the field of preparation of fat emulsion for clevidipine butyrate injection, can solve problems such as few detailed reports, and achieves stable quality, stable preparation process and convenient use. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Single-dose prescription composition
[0025] Element
Dosage (mg)
Clevidipine Butyrate
25
10000
600
15
glycerin
1125
2.5
5
Water for Injection
up to 50ml
[0026] Preparation:
[0027] Dosing process:
[0028] Oil phase preparation: under the protection of nitrogen, add the prescribed amount of soybean oil, egg yolk lecithin and oleic acid into the liquid mixing tank A, stir and heat to 60°C, keep warm, add the prescribed amount of clevidipine butyrate, and high-speed (2500r / min) and stir until the clevidipine butyrate is completely dissolved.
[0029] Water phase preparation: Add the prescribed amount of glycerin, edetate disodium and water for injection into the liquid mixing tank B, stir to completely dissolve and mix evenly, control the liquid mixing temperature at 60°C, and keep warm.
[...
Embodiment 2
[0037] Single-dose prescription composition
[0038] Element
Dosage (mg)
Clevidipine Butyrate
25
10000
egg yolk lecithin
600
15
glycerin
1125
2.5
5
Water for Injection
up to 50ml
[0039] Preparation:
[0040] Dosing process:
[0041] Oil phase preparation: under the protection of nitrogen, add the prescribed amount of soybean oil, egg yolk lecithin and oleic acid into the liquid mixing tank A, stir and heat to 60°C, keep warm, add the prescribed amount of clevidipine butyrate, and high-speed (2500r / min) and stir until the clevidipine butyrate is completely dissolved.
[0042] Water phase preparation: Add the prescribed amount of glycerin, edetate disodium and water for injection into the liquid mixing tank B, stir to completely dissolve and mix evenly, control the liquid mixing temperature at 60°C, and keep warm.
[...
Embodiment 3
[0050] Single-dose prescription composition
[0051] Element
Dosage (mg)
Clevidipine Butyrate
25
10000
egg yolk lecithin
600
15
glycerin
1125
2.5
5
Water for Injection
up to 50ml
[0052] Preparation:
[0053] Dosing process:
[0054] Oil phase preparation: under the protection of nitrogen, add the prescribed amount of soybean oil, egg yolk lecithin and oleic acid into the liquid mixing tank A, stir and heat to 60°C, keep warm, add the prescribed amount of clevidipine butyrate, and high-speed (2500r / min) and stir until the clevidipine butyrate is completely dissolved.
[0055] Water phase preparation: Add the prescribed amount of glycerin, edetate disodium and water for injection into the liquid mixing tank B, stir to completely dissolve and mix evenly, control the liquid mixing temperature at 60°C, and keep warm.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com