Injection of serine protease inhibitor
A technology of serine protease and injection, applied in the field of biopharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0041] Example 1 Preparation of Recombinant textilinin-1
[0042] Recombinant textilinin-1 involved in this patent is a recombinant protein, which is prepared by the method disclosed in the patent application number 201110178605.6. The specific preparation method is as follows:
[0043] The amino acid sequence of Recombinant textilinin-1 involved in the present invention is: SEQ NO 1: KDRPDFCELPADTGPCRVRFPSFYYNPDEKKCLEFIYGGCEGNANNFITKEECESTCAA.
[0044] The artificially synthesized textilinin-1 structural gene is obtained by secreting and expressing a large amount of it in Pichia pastoris. According to the natural amino acid sequence of textilinin-1, the preferred codon of methanolic yeast is selected to artificially synthesize textilinin-1 Structural gene sequence, the gene sequence is: SEQ NO 2: AAG GAT AGA CCA GAT TTT TGT GAA TTG CCA GCT GAT ACT GGT CCA TGT AGA GTT AGA TTT CCA TCT TTT TAC TAC AAC CCA GAT GAA AAG AAG TGT TTG GAA TTT ATT TAC GGT GGT TGT GAA GGT AAC GCT AA...
Embodiment 4
[0057] Example 4 Selection of pH and concentration range of suitable buffer system for Recombinant textilinin-1
[0058] (1) Screening of the pH of the applicable buffer system
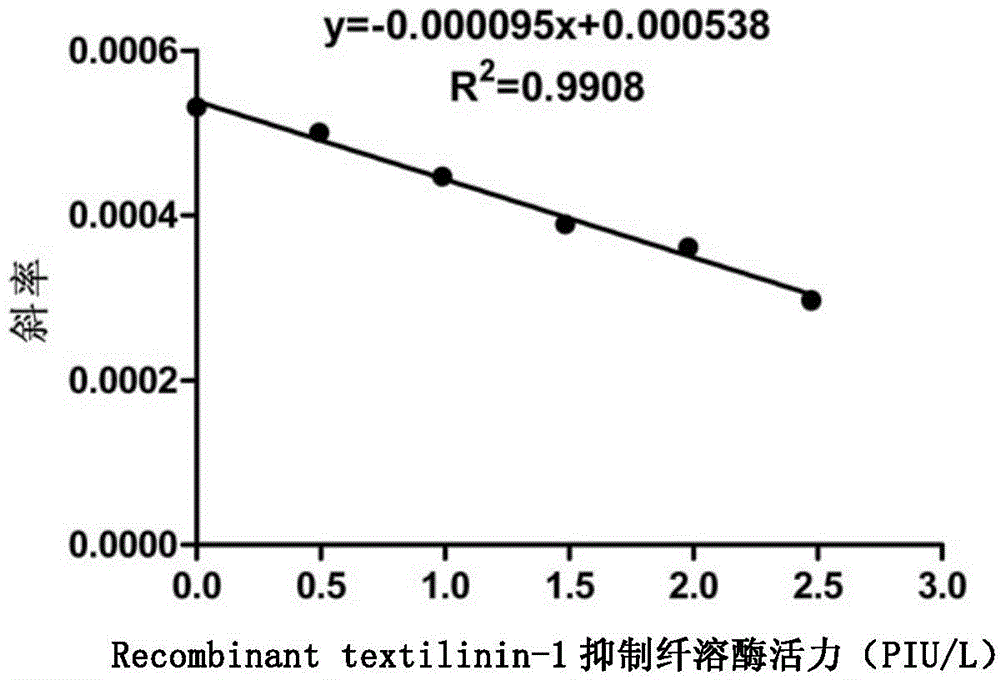
[0059] Randomly select the Recombinant textilinin-1 concentrate produced by Liaoning Nuokang Biopharmaceutical Co., Ltd., and use 50mmol / L Tris-HCl or 50mmol / L Na 2 HPO 4 -NaH 2 PO 4 Phosphate is prepared into diluent (the preparation method of Tris-HCl buffer solution and phosphate buffer saline solution is as shown in Table 2), draws the Recombinant textilinin-1 dilution solution to be tested and is placed in cuvette for activity measurement, the results are shown in figure 2 .
[0060] Table 2 Preparation of Tris-HCl buffer and phosphate buffer
[0061]
[0062]
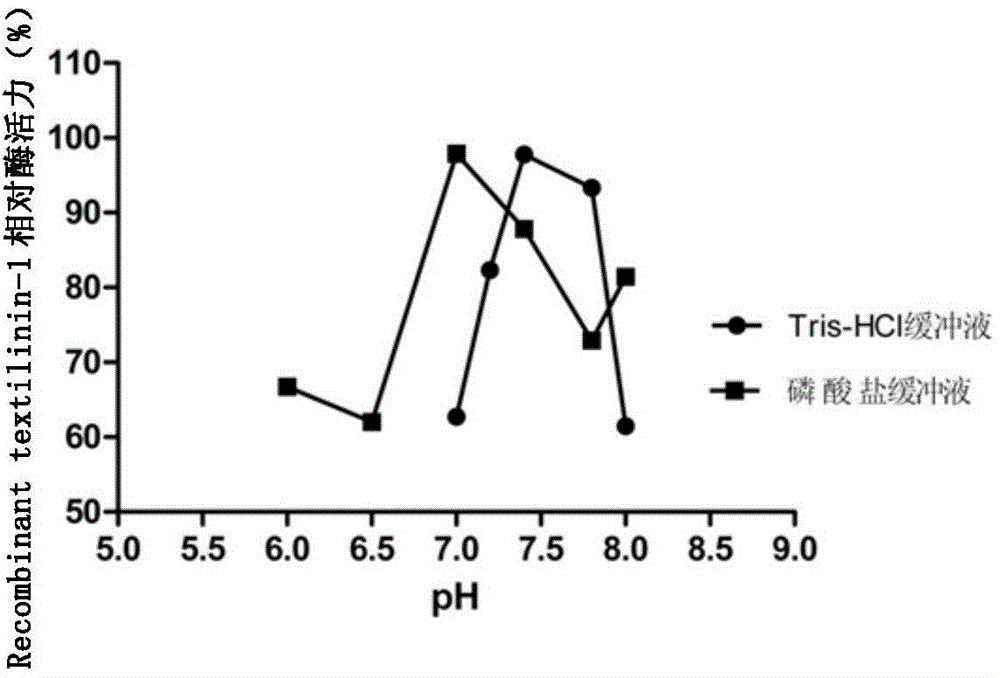
[0063] like image 3 As shown, the relative enzyme activity of Recombinant textilinin-1 is affected by the pH value of the buffer, wherein, when the pH of Tris-HCl buffer is 7.2-7.8, Recombinant textilinin-1 has a higher e...
Embodiment 5
[0067] Example5 Effects of Anions on the Stability of Recombinant textilinin-1
[0068] (1) Effect of anion species on the stability of Recombinant textilinin-1
[0069] Take different kinds of anions and dilute them with 50mmol / L Tris-HCl diluent of pH 7.4 so that 1mg / ml Recombinant textilinin-1 contains 50mmol / L anions, and place them at a temperature of 60°C for about 18 hours, Measure again, dilute in 1ml reaction system, make Recombinant textilinin-1 reaction final concentration be 5nmol / L, carry out activity measurement in ultraviolet spectrophotometer, the Recombinant textiliinin-1 of different anions measures activity respectively before and after heating, this Recombinant The relative enzyme activity of textilinin-1 is the ratio of the activity of the same kind of anion after heating to the activity before heating. In order to remove the influence of anions on plasmin, measure the activity of plasmin with the same concentration of anions, the blank is the diluent, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com