Fluorine-doped carbon-coated positive electrode composite material and preparation method and application thereof
A technology of composite material and positive electrode material, which is applied in the field of fluorine-doped carbon-coated positive electrode composite material and its preparation, can solve the problems of inability to prevent electrolyte, low conductivity, poor uniformity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
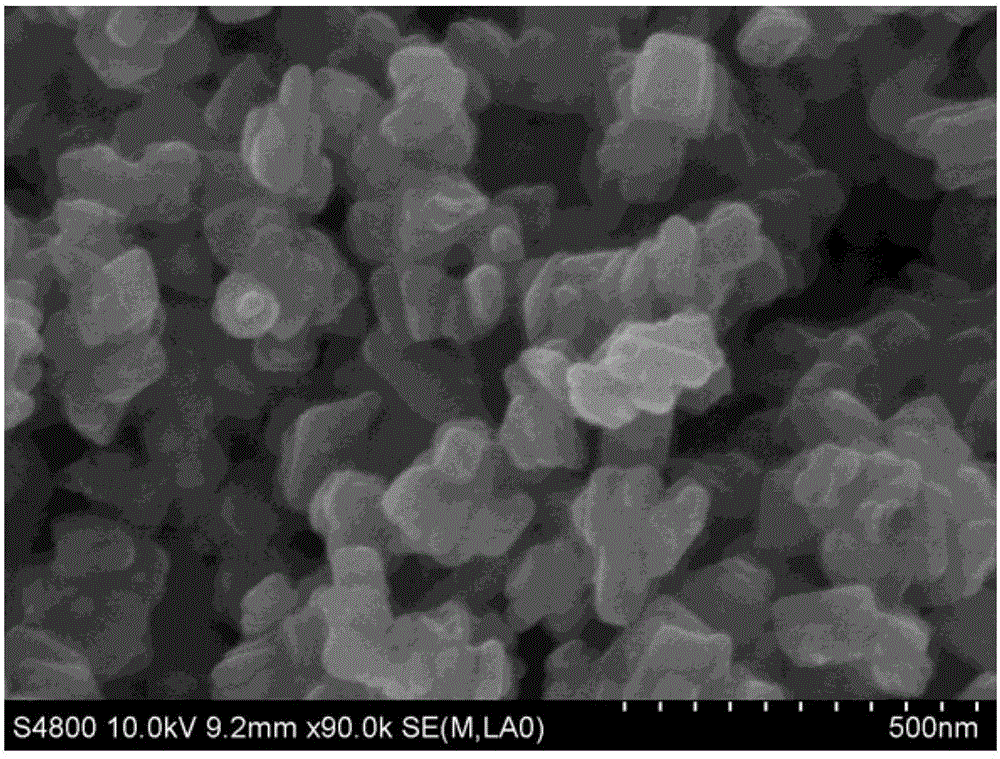
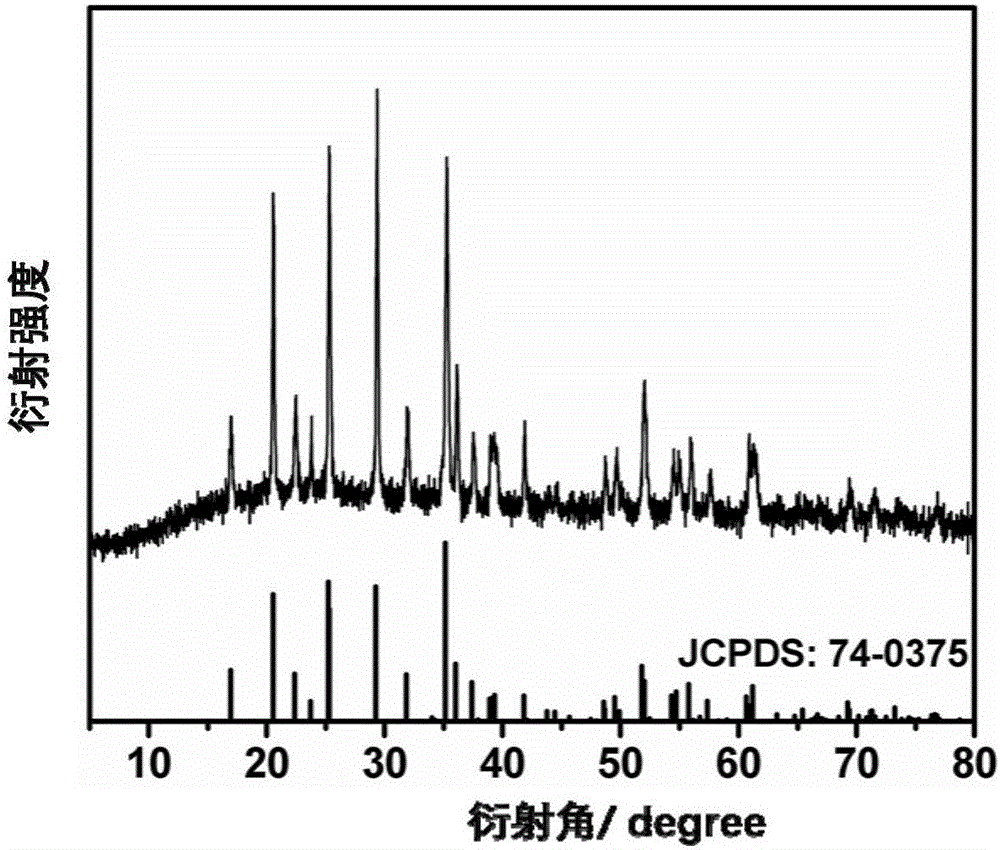
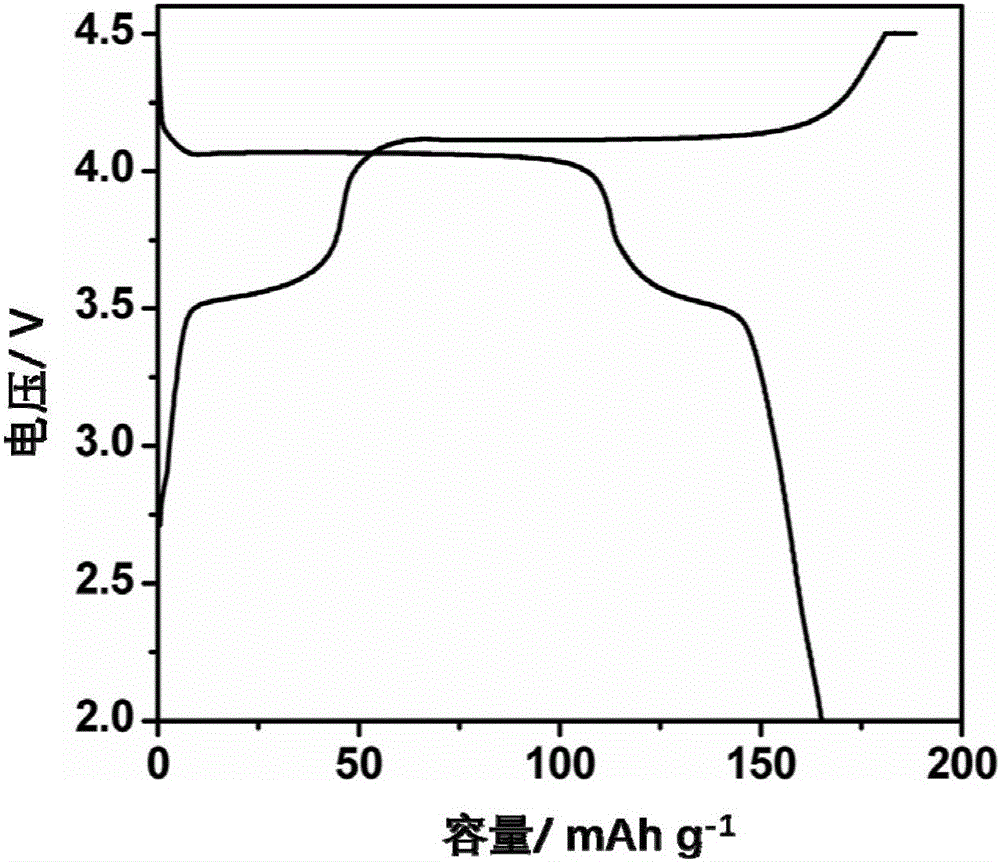
[0037] Water and diethylene glycol are configured as a mixed solvent in a ratio of 1:2. A stoichiometric lithium salt and a stoichiometric phosphorus source were added to a mixed solvent of water and alcohol, and stirred for 30 minutes to form a suspension A. Add stoichiometric ratio of ferrous salt, stoichiometric ratio of divalent manganese salt and 0.2 g of ascorbic acid into the mixed solvent of water and alcohol, and stir for 10 minutes to form solution B. Solution B was added dropwise to suspension A shown, and stirred for 10 min to form suspension C. Wherein Li:Mn:Fe:P:=3:0.75:0.25:1. The suspension C was transferred to the reaction kettle, and the solvothermal reaction was carried out at 200 ° C for 12 hours to obtain lithium manganese iron phosphate (LiMn 0.75 Fe 0.25 PO 4 ) precursor slurry. The lithium manganese iron phosphate slurry was naturally cooled to room temperature, and washed alternately with water and ethanol three times. The washed product was plac...
Embodiment 2
[0041] Water and diethylene glycol are configured as a mixed solvent in a ratio of 1:2. A stoichiometric lithium salt and a stoichiometric phosphorus source were added to a mixed solvent of water and alcohol, and stirred for 30 minutes to form a suspension A. Add stoichiometric ratio of ferrous salt, stoichiometric ratio of divalent manganese salt and 0.2 g of ascorbic acid into the mixed solvent of water and alcohol, and stir for 10 minutes to form solution B. Solution B was added dropwise to suspension A shown, and stirred for 10 min to form suspension C. Wherein Li:Mn:Fe:P:=3:0.75:0.25:1. The suspension C was transferred to the reaction kettle, and the solvothermal reaction was carried out at 200 ° C for 12 hours to obtain lithium manganese iron phosphate (LiMn 0.75 Fe 0.25 PO 4 ) precursor slurry. The lithium manganese iron phosphate slurry was naturally cooled to room temperature, and washed alternately with water and ethanol three times. The washed product was plac...
Embodiment 3
[0044] Water and diethylene glycol are configured as a mixed solvent in a ratio of 1:2. A stoichiometric lithium salt and a stoichiometric phosphorus source were added to a mixed solvent of water and alcohol, and stirred for 30 minutes to form a suspension A. Add stoichiometric ratio of ferrous salt, stoichiometric ratio of divalent manganese salt and 0.2 g of ascorbic acid into the mixed solvent of water and alcohol, and stir for 10 minutes to form solution B. Solution B was added dropwise to suspension A shown, and stirred for 10 min to form suspension C. Wherein Li:Mn:Fe:P:=3:0.75:0.25:1. The suspension C was transferred to the reaction kettle, and the solvothermal reaction was carried out at 200 ° C for 12 hours to obtain lithium manganese iron phosphate (LiMn 0.75 Fe 0.25 PO 4 ) precursor slurry. The lithium manganese iron phosphate slurry was naturally cooled to room temperature, and washed alternately with water and ethanol three times. The washed product was plac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Reversible specific capacity | aaaaa | aaaaa |
| Reversible specific capacity | aaaaa | aaaaa |
| Reversible specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com