Cysteine engineered fibronectin type III domain binding molecules
A cysteine, fibronectin technology, applied in the direction of peptide/protein components, immunoglobulins, animal/human proteins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0364] Example 1: Construction of Tencon library
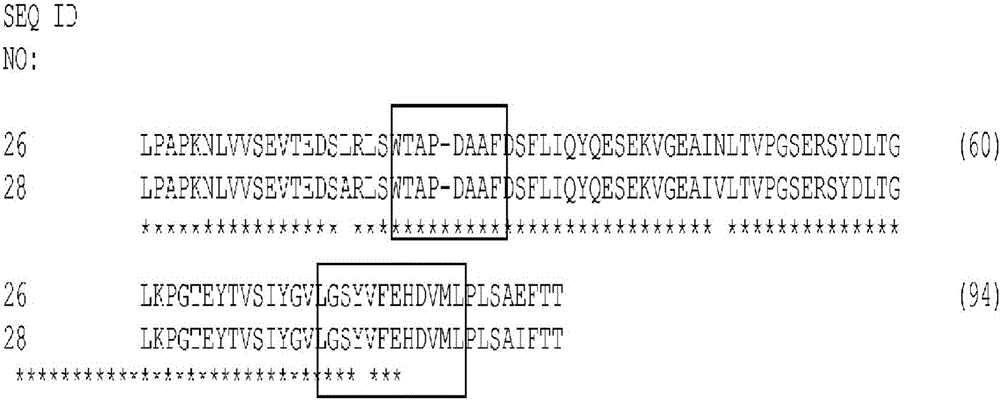
[0365] Tencon (SEQ ID NO: 1) is an immunoglobulin-like scaffold designed from the consensus sequence of fifteen FN3 domains from human tenascin-C fibronectin (FN3) domain type III (Jacobs et al., Protein Engineering, Design, and Selection, 25:107-117, 2012; US Patent Publication 2010 / 0216708). The crystal structure of Tencon shows six surface-exposed loops linking seven β-strands. Selected residues within the or each loop can be randomized in order to construct a type III fibronectin (FN3) domain library that can be used to select novel molecules that bind specific targets.
[0366] Tencon:
[0367] LPAPKNLVVSEVTEDSLRLSWTAPDAAFDSFLIQYQESEKVGEAINLTVPGSERSYDLTGLKPGTEYTVSIYGVKGGHRSNPLSAEFTT (SEQ ID NO 1):
[0368] Construction of TCL1 library
[0369] A library TCL1 designed to randomize only the FG loop of Tencon (SEQ ID NO: 1) was constructed for use with the cis-display system (Jacobs et al., Protein Engineering, Desig...
example 2
[0391] Example 2: Selection of Type III Fibronectin (FN3) Domains that Bind EGFR and Inhibit EGF Binding
[0392] library screening
[0393] Cis display was used to select EGFR binding domains from TCL1 and TCL2 libraries. The recombinant human extracellular domain of EGFR fused to IgGl Fc (R&D Systems) was biotinylated using standard methods and used for panning (residues 25-645 of full-length EGFR of SEQ ID NO: 73). For in vitro transcription and translation (ITT), 2-6 μg of library DNA was incubated with 0.1 mM intact amino acids, 1X S30 master mix components and 30 μL S30 extract (Promega) in a total volume of 100 μL and incubated at 30°C . After 1 hour, 450 μL of blocking solution (PBS pH 7.4 supplemented with 2% bovine serum albumin, 100 μg / mL herring sperm DNA and 1 mg / mL heparin) was added and the reaction was incubated on ice for 15 minutes. EGFR-Fc was assembled at 1:1 and 10:1 molar ratios of EGFR to EGF by mixing recombinant human EGF (R&D Systems) and bioti...
example 3
[0411] Example 3: Characterization of EGFR-binding FN3 domains that inhibit EGF binding
[0412] Large scale expression, purification and endotoxin removal
[0413] The nine FN3 domains shown in Table 4 were enlarged to provide more material for detailed characterization. Using overnight cultures containing each EGFR-binding FN3 domain variant, dilute the overnight culture 1 / 80 with 0.8 L of Terrific broth medium supplemented with 100 μg / mL ampicillin and inoculate into fresh medium, Incubate with shaking at 37°C. When the optical density at 600 nm reached about 1.2-1.5 by adding IPTG to a final concentration of 1 mM, the culture was induced and the temperature was lowered to 30°C. After 4 hours, cells were collected by centrifugation and the cell pellet was stored at -80°C until required for use.
[0414] For cell lysis, dissolve the thawed pellet at 5 mL per gram of pellet The ratio was resuspended in supplemented with 25U / mL (Sigma-Aldrich) and 1kU / mL rLysozyme ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com