A block anion exchange membrane and its preparation method
A technology of anion exchange membrane and block polymer, which is applied in the field of new block anion exchange membrane and its preparation, can solve the problems of hindered molecular chain rotation, increased internal stress of the membrane, and brittle dry film, etc., so as to alleviate mechanical strength, Effects of improving mechanical strength and increasing free volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
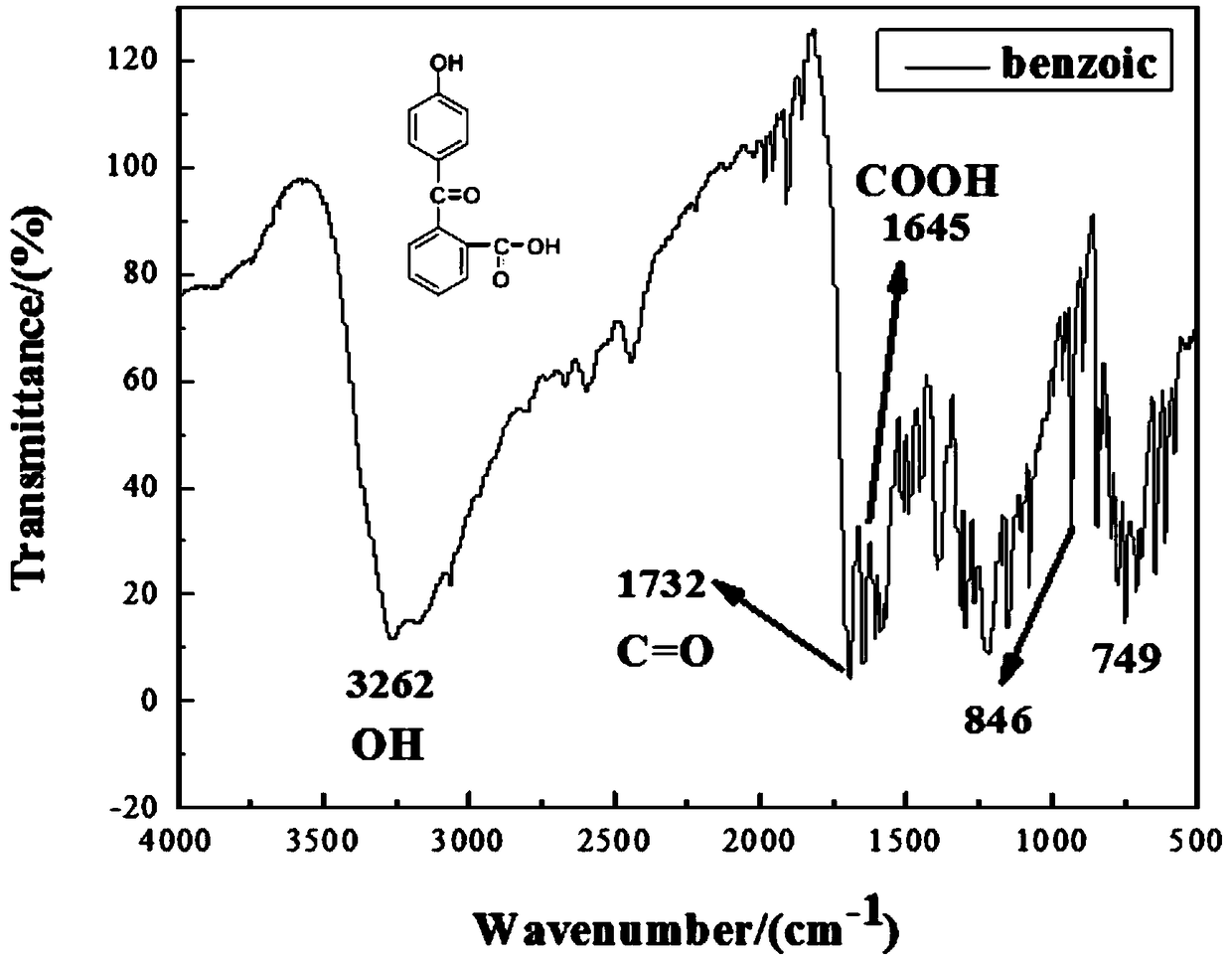
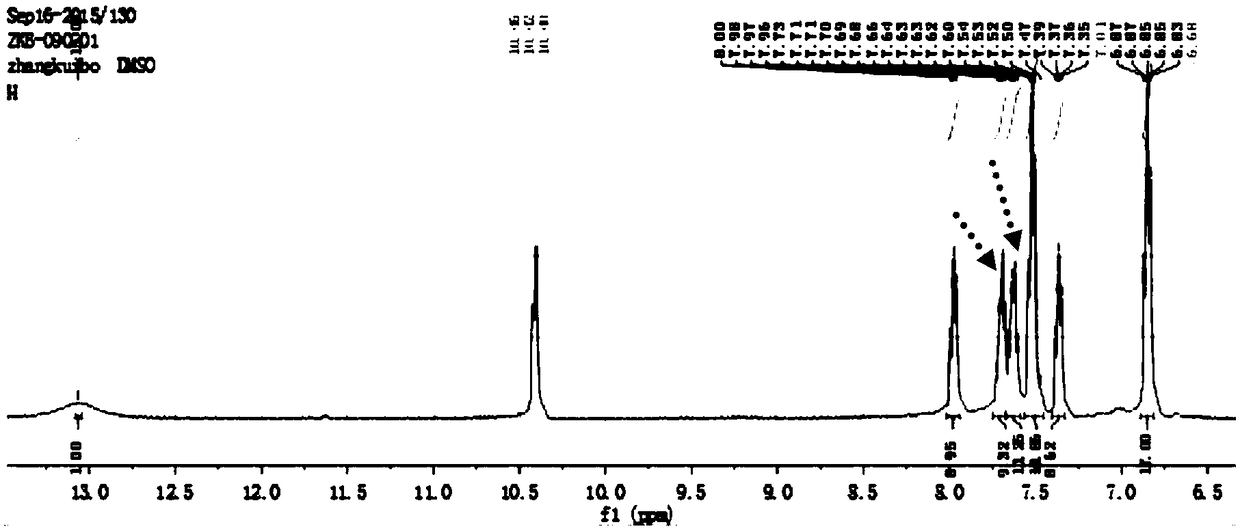
[0033] Dissolve phenolphthalein and hydroxylamine hydrochloride in KOH solution (phenolphthalein: hydroxylamine hydrochloride = 10 mmol: 1.05 mmol), where the concentration of phenolphthalein is 1.2 mol / L, react at 80°C for 3-4 hours to obtain a reaction solution; pour the liquid into acetic acid Acidification treatment in the aqueous solution, filter to remove the insoluble matter to obtain a mixed solution; Add the KOH aqueous solution to the mixed solution until the pink color disappears, stop adding the KOH solution, continue the reaction for about 10 minutes, and then add an appropriate amount of absolute ethanol as a dispersant, and use acetic acid to make The solution was weakly acidic; the yellow substance was filtered and dissolved in an aqueous sulfuric acid solution and refluxed for about 2 hours. After cooling, a gray-green solid was obtained, namely 2-(4-hydroxybenzoyl)-benzoic acid.
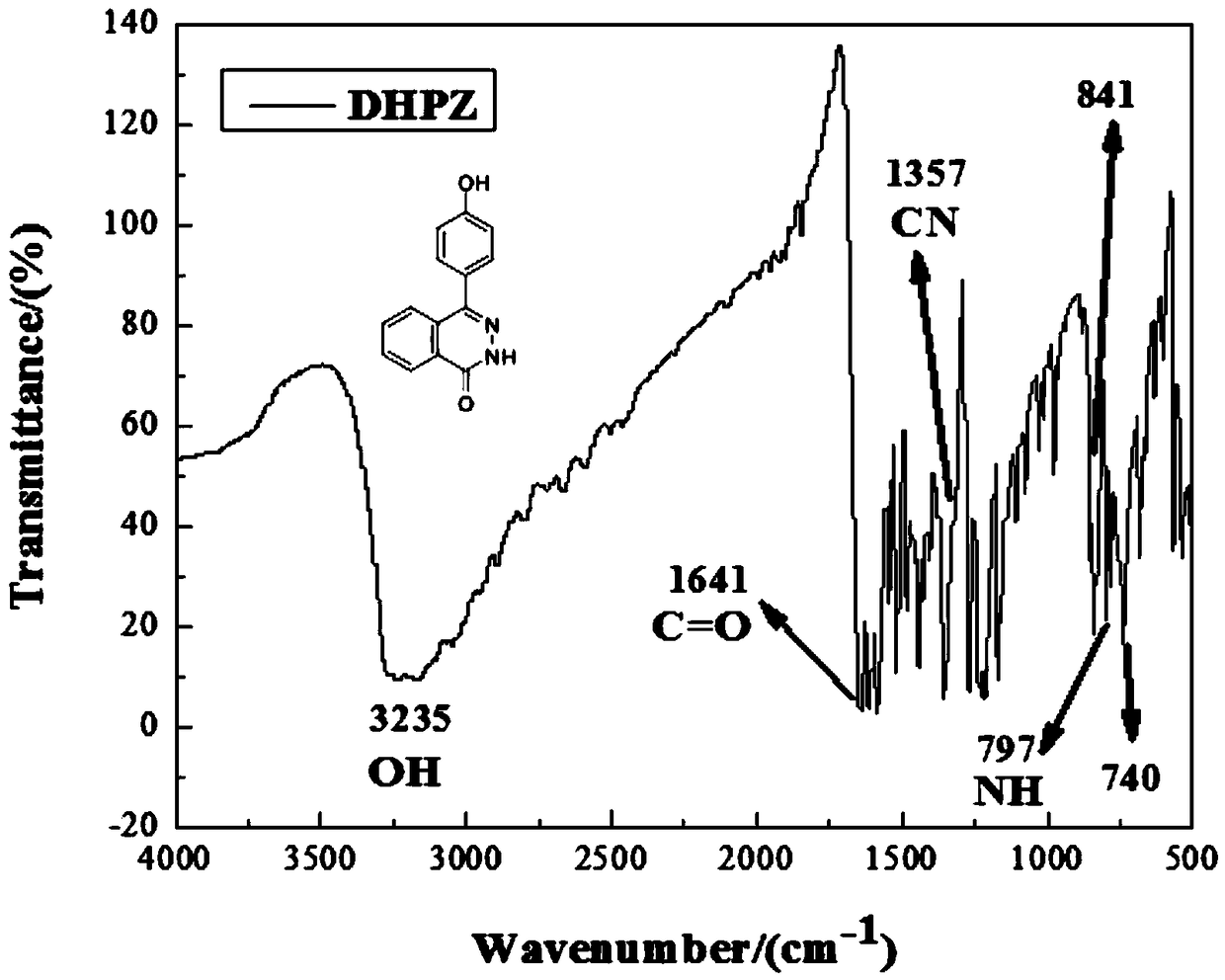
[0034] The 2-(4-hydroxybenzoyl)-benzoic acid and hydrazine hydrate prepared above w...
Embodiment 2
[0043] At room temperature, dissolve decafluorobiphenyl and bisphenol fluorene in N,N-dimethylacetamide, add potassium carbonate as a catalyst and toluene as a dewatering agent, heat up to 60°C and react for 12 hours, then pour the reacted liquid Precipitation into water / ethanol and mixed precipitation agent to obtain oligomer 1 (decafluorobiphenyl: bisphenol fluorene: potassium carbonate = 20mmol: 18.4mmol: 36.8mmol), wherein the molar concentration of decafluorobiphenyl is 0.13mol / L;
[0044] At room temperature, dissolve decafluorobiphenyl and 4-(4-hydroxyphenyl)-2,3-phthalazin-1-one (DHPZ) in N,N-dimethylacetamide, where DHPZ is Prepared in Example 1, and added the catalyst potassium carbonate and the dewatering agent toluene, heated to 50 ℃ and reacted for 12 hours, the reacted liquid was poured into water / ethanol and mixed precipitation agent to precipitate to obtain oligomer 2 (decafluoro Biphenyl: bisphenol fluorene: potassium carbonate = 18mmol: 21.6mmol: 43.2mmol), wher...
Embodiment 3
[0050] At room temperature, dissolve decafluorobiphenyl and bisphenol fluorene in N,N-dimethylacetamide, add potassium carbonate as a catalyst and toluene as a dewatering agent, heat up to 65°C and react for 12 hours, then pour the reacted liquid Precipitation into water / ethanol and mixed precipitation agent to obtain oligomer 1 (decafluorobiphenyl: bisphenol fluorene: potassium carbonate = 19.5mmol: 17.94 mmol: 35.88mmol), in which the molar concentration of decafluorobiphenyl is 0.14mol / L ;
[0051] At room temperature, dissolve decafluorobiphenyl and 4-(4-hydroxyphenyl)-2,3-phthalazin-1-one (DHPZ) in N,N-dimethylacetamide, where DHPZ is Prepared in Example 1, and added the catalyst potassium carbonate and the dewatering agent toluene, heated to 50 ℃ and reacted for 12 hours, the reacted liquid was poured into water / ethanol and mixed precipitation agent to precipitate to obtain oligomer 2 (decafluoro Biphenyl: bisphenol fluorene: potassium carbonate = 15mmol: 18mmol: 36mmol), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com