Alkaline electrolyte and method for preparing bright nickel by means of electrolysis in alkaline electrolyte
An electrolyte and bright nickel technology, applied in the field of non-ferrous metallurgy, can solve the problems of nickel blackening and brittleness, and achieve good toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
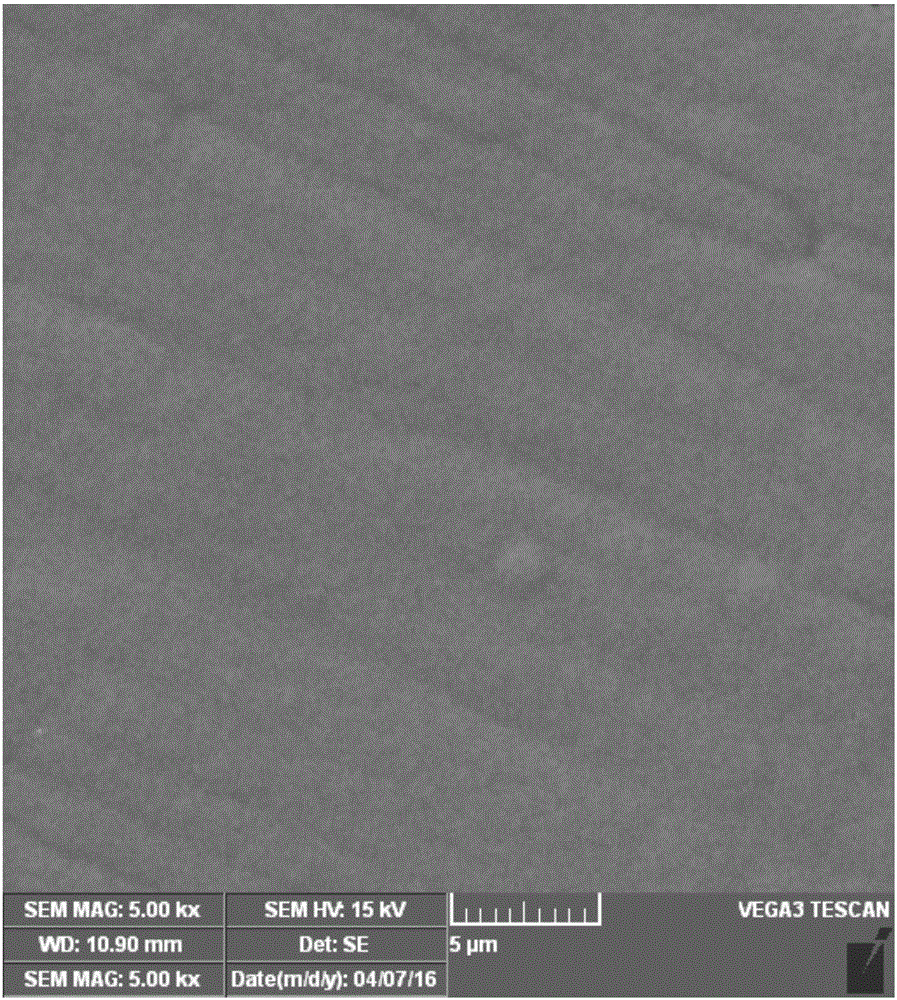
[0033] Configure 250 mL of electrolyte solution containing 50 g / L of nickel chloride, 80 g / L of ammonium chloride, 30 g / L of ammonia water, and 0.03 g / L of sodium benzenesulfinate. Take the anode of the thick nickel electrode and immerse it in 1mol / L nitric acid for 60 minutes to remove the surface oxide layer and impurities, take it out, rinse it with deionized water, and dry it with cold air. Using stainless steel as the cathode material, after grinding with 1#, 3#, and 5# sandpaper, degreasing with absolute ethanol, washing with deionized water and drying with cold air, put the cathode and anode into the electrolytic cell at the same time, and control the electrodeposition temperature to 25℃, the current density is -500A / m 2 , Stir the electrolyte at a speed of 650r / min. After 0.5h of electrodeposition, take out the cathode, rinse the residual electrolyte on the surface with water, dry it with cold air, and peel off the nickel from the stainless steel to obtain high-purity ...
Embodiment 2
[0036] Prepare 250 mL of electrolyte solution containing 400 g / L of nickel chloride, 450 g / L of ammonium chloride, 350 g / L of ammonia water, and 1 g / L of sodium benzenesulfinate. Take the anode of the thick nickel electrode, soak it in 5mol / L nitric acid for 10min to remove the surface oxide layer and impurities, take it out, rinse it with deionized water, and dry it with cold air. Using stainless steel as the cathode material, after grinding with 1#, 3#, and 5# sandpaper, degreasing with absolute ethanol, washing with deionized water and drying with cold air, put the cathode and anode into the electrolytic cell at the same time, and control the electrodeposition temperature to 65℃, the current density is -100A / m 2 , Stir the electrolyte at a speed of 250r / min. After 100 hours of electrodeposition, take out the cathode, rinse the residual electrolyte on the surface with water, dry it with cold air, and peel off the nickel from the stainless steel to obtain high-purity nickel w...
Embodiment 3
[0039] Configure 250 mL of electrolyte containing 80 g / L of nickel chloride, 100 g / L of ammonium chloride, 50 g / L of ammonia water, and 0.05 g / L of sodium benzenesulfinate. Take the anode of the thick nickel electrode and immerse it in 2mol / L nitric acid for 40min to remove the surface oxide layer and impurities, take it out, rinse it with deionized water, and dry it with cold air. Using stainless steel as the cathode material, after grinding with 1#, 3#, and 5# sandpaper, degreasing with absolute ethanol, washing with deionized water and drying with cold air, put the cathode and anode into the electrolytic cell at the same time, and control the electrodeposition temperature to 40℃, the current density is -400A / m 2 , Stir the electrolyte at a speed of 500r / min. After 1 hour of electrodeposition, take out the cathode, rinse the residual electrolyte on the surface with water, dry it with cold air, and peel off the nickel from the stainless steel to obtain high-purity nickel with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com